




How Do Different Types of Crystals Form?
Crystals have been defined in many ways. The most common definition of crystals is a solid substance in which atoms are repeated regularly in three dimensions. It's a substance having a regular 3-dimensional shape with a definite geometry and plane surface.
Different Crystals
Types of Crystals and Their Features
Ionic Crystals:- Their constituent particles are cations and negative ions. These ions are held together by an electro-attractive force.
Main Features of Ionic Crystals:-
They are solid and brittle.
Their melting and boiling points are very high.
In solid-state, they do not conduct electricity.
They dissolve easily in water and other polar solvents.
Covalent Crystals:- Many crystalline solids of non-metals are produced by forming covalent bonds between adjacent atoms. Covalent solids are large molecules.
Main Characteristics of Covalent Crystals:-
These are often very hard.
Due to the directional nature of the binding, they do not occupy maximum space.
Their melting and boiling points are very high.
Their frequency index is very high.
They do not conduct electricity, except for graphite.
They absorb light with different vibrations.
Molecular Crystals:- They have molecules at their lattice points, on which there is no charge. Weak Van der Waals force acts in such a solid substance which is the blood by binding the atoms and molecules together.
Key Features of Molecular Crystals:-
They are volatile.
Their melting and boiling points are low.
Their vapour heat is very low.
In the solid state, these crystals are semi-transparent, but in the liquid state, they are transparent.
Metallic Crystals:- Atoms in metallic crystals are held together by metallic bonds. The valence electrons of the metal atom are released and are covered by the cation. These free electrons move freely in the spaces between the cations.
Key Features of Metallic Crystals:-
Metals are good conductors of heat and electricity due to the moving electrons present in metal crystals.
Metals are non-metallic and ductile.
When a voltage is applied between the two ends of the metal, the electrons start passing through the metal ions, which is why metals are good conductors of heat and electricity.
A special type of lustre is found in metals called metallic lustre.
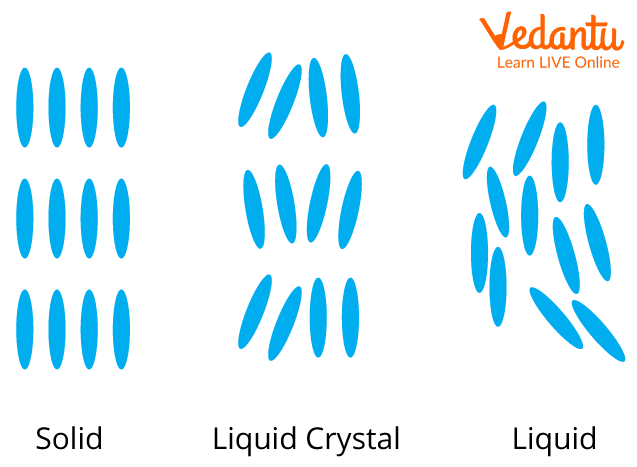
What are Liquid Crystals?
Liquid crystal is a term referring to those that are not crystalline (solid) nor isotropic (liquid), but somewhere in between the two. Three main types of liquid crystals can be identified by their varying amounts of molecular order and position. This arrangement of molecules is what makes a substance more solid or liquid.
Types of Liquid Crystals
In studies, we find that there can be many types of liquid crystalline states based on optical properties. Liquid crystal states can be divided into thermotropic, lyotropic and metallotropic, etc. The molecules found in thermotropic and lyotropic are of organic type. When the temperature changes, the state transitions from the thermotropic liquid crystal to the liquid crystal state.
Types of Liquid Crystals
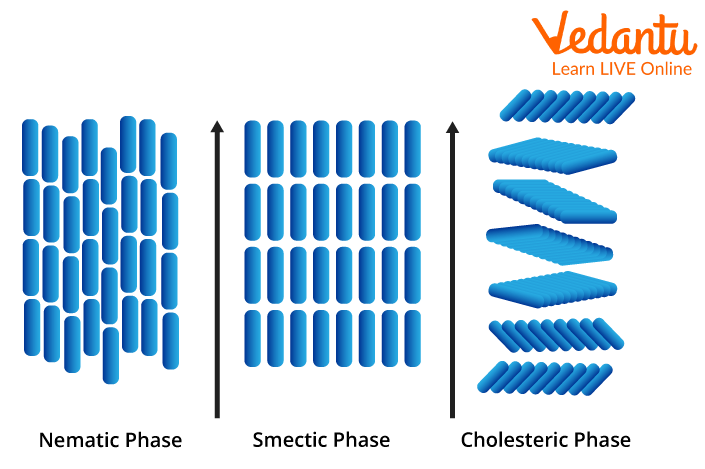
● Nematic: The nematic phase is the simplest form of liquid crystal and is the phase in which the crystal molecules have no arranged positions and are free to move in any way they like. However, while they do not have a specific order, the molecules point in the same direction during this phase, distinguishing them from a pure liquid.
The liquid crystal at this stage can be characterised by its thread-like appearance when viewed under a microscope. The use of nematic liquid crystals in telescope lenses is common because it allows for a clearer image when researchers are faced with atmospheric turbulence.
● Smectic: In this step, the molecules line up in layers, keeping the same orientation and pointing in the same direction as the molecules in nematic liquid crystals. While these layers move freely, movement within the layers is restricted; Therefore, it forms a slightly more solid substance. Smectic liquid crystals have been found to have fast electro-optical response times and are therefore used in conjunction with nematic liquid crystals in making liquid crystal display (LCD) screens.

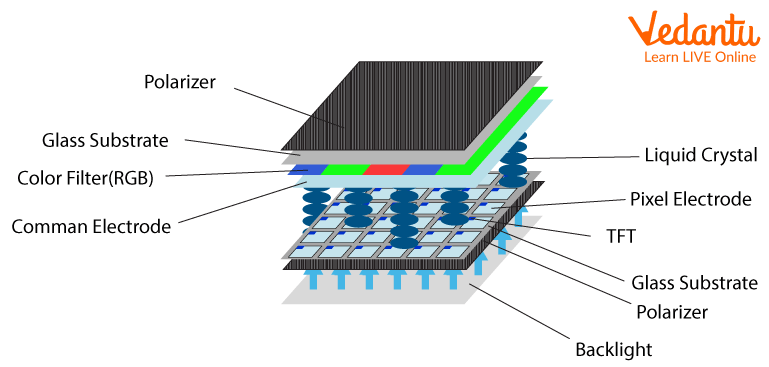
LCD
● Cholesteric: The cholesteric phase, also known as the chiral nematic phase, is characterised by molecules being aligned and stacked at a slight angle to each other within very thin layers – this prevents a substance from being crystalline or solid. This type of liquid crystal also has the characteristic of changing colour when exposed to different temperatures. This is why cholesteric liquid crystals are used in common household items such as thermometers and mood rings.
Types of Crystals in the Urine
The Types of Urine Crystals are
1. Struvite - The problem with struvite is the high level of phosphate, ammonium, magnesium and calcium in the body.
2. Uric Acid Stones – They are caused by acidic urine.
3. Calcium Oxalate - According to a report published in the National Kidney Foundation, calcium oxalate is the main cause of kidney stone problems.
4. Cystine Stones - Cystine stones are larger than kidney stones.
Urine Crystals

Kidney Stone
Solved Questions
1. What is a liquid crystal, and how many types are there with applications?
Ans: Liquid Crystal Types and Applications: Some organic crystalline solids, on heating, attain a liquid-like state in a certain temperature range, but their molecules are oriented in a certain direction, so they maintain asymmetrically directional properties; such instances are called liquid crystals. These crystals are used in LCD (liquid crystal display).
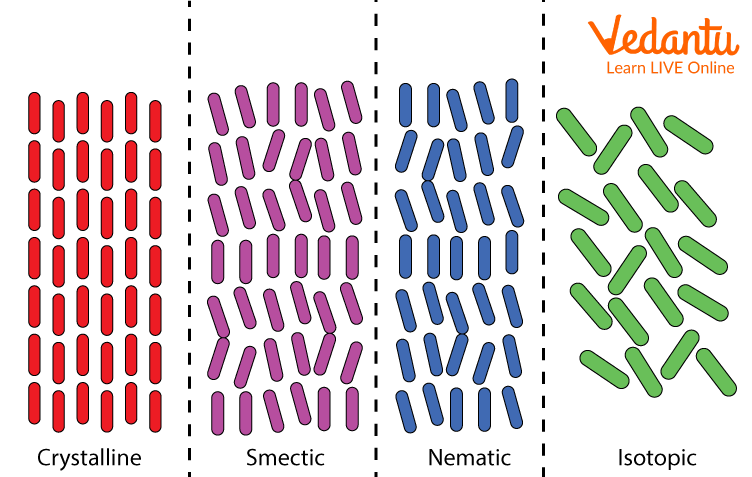
Types of Liquid Crystals
2. What is the difference between nematic, smectic and cholesteric liquid crystals?
Ans: The key difference between nematic smectic and cholesteric liquid crystals are their structure. The molecules in nematic liquid crystals have no ordered structure and smectic liquid crystals have a layered molecular structure, while the molecules in cholesteric liquid crystals are in a twisted and chiral arrangement.
Learning by Doing
Write True or False.
1. LCDs' full form is Liquid Crystal Displays.
2. LCDs are used in making photo frames.
3. Liquid Crystals are used in LCD.
4. Nematic liquid crystal is a type of metallotropic liquid crystal.
Summary
Liquid crystal is the state of any substance whose properties are found between that of the original solid and that of the liquid; that is, it may have some properties of the solid, and some properties may be present in the liquid. Liquid crystals are used in LCD (liquid crystal display). LCDs are used in electronic clocks and microcomputers. There are three types of thermotropic liquid crystals- Nematic, Smectic and Cholesteric Liquid Crystals.
FAQs on Types of Crystals: Key Concepts and Examples
1. What are the basic properties that define a crystal?
A crystal is a solid material whose atoms, molecules, or ions are arranged in a highly ordered, repeating pattern called a crystal lattice. Key properties include:
Definite Geometric Shape: Crystals have flat faces and sharp, well-defined edges.
Uniform Composition: They are homogenous, meaning they are uniform throughout.
Sharp Melting Point: Unlike amorphous solids, crystals melt at a specific, fixed temperature.
Anisotropy: Their physical properties, like electrical conductivity or refractive index, can vary with direction.
2. What is the main difference between crystalline and amorphous solids?
The primary difference lies in the internal arrangement of their constituent particles. Crystalline solids have a long-range, orderly, and repeating three-dimensional arrangement of atoms. In contrast, amorphous solids (like glass or plastic) have a disordered, random arrangement of atoms with no long-range order.
3. On what basis are the different types of crystals classified?
Crystals are classified based on their internal structure, specifically the geometry of their unit cell. A unit cell is the smallest repeating unit of a crystal lattice. The classification depends on the lengths of the unit cell's edges (a, b, c) and the angles between them (α, β, γ). This gives rise to seven unique crystal systems.
4. What are the seven main types of crystal systems?
The seven fundamental crystal systems, based on their atomic lattice structure, are:
Cubic: All sides and angles are equal (e.g., Salt, Diamond).
Tetragonal: Two sides are equal, all angles are 90° (e.g., Zircon).
Orthorhombic: Unequal sides, all angles are 90° (e.g., Topaz).
Monoclinic: Unequal sides, two angles are 90° (e.g., Gypsum).
Triclinic: All sides and angles are unequal (e.g., Turquoise).
Hexagonal: Two sides equal, specific angles of 90° and 120° (e.g., Quartz, Graphite).
Trigonal (or Rhombohedral): All sides equal, all angles equal but not 90° (e.g., Calcite).
5. Why do different crystals, like salt and quartz, have completely different shapes?
The external shape of a crystal is a direct reflection of its internal atomic arrangement. Salt (Sodium Chloride) has a cubic unit cell, which, when repeated millions of times, results in a cube-shaped crystal. Quartz has a hexagonal unit cell, leading to its characteristic six-sided prism shape. Therefore, the unique geometry of the underlying crystal lattice dictates the final macroscopic shape of the crystal.
6. What are some important examples of crystals in everyday technology?
Crystals are crucial in many modern technologies. For example:
Silicon crystals are the foundation of all electronics, including computer chips and processors.
Quartz crystals are used in watches and clocks for precise timekeeping due to their piezoelectric properties.
Liquid crystals are used in displays for TVs, laptops, and calculators (LCDs).
Diamond crystals, due to their hardness, are used in industrial cutting and drilling tools.
7. What makes some crystals, like diamond, exceptionally hard?
A crystal's hardness is determined by the strength of the chemical bonds holding its atoms together and how tightly these atoms are packed. In a diamond, each carbon atom is bonded to four other carbon atoms with extremely strong covalent bonds in a rigid, three-dimensional lattice. This structure makes it very difficult to scratch or break the crystal, placing it at 10 on the Mohs scale of hardness, the highest possible value.
8. What are liquid crystals and what makes them unique?
Liquid crystals represent a unique state of matter that has properties of both conventional liquids and solid crystals. Like a liquid, their molecules can flow. However, like a crystal, their molecules are arranged in an ordered, crystal-like way. This dual nature is why they are useful in Liquid Crystal Displays (LCDs), as their molecular orientation can be controlled by applying an electric field, which in turn affects how light passes through them to create an image.









