




Key Discoveries and Impact of Rutherford in Atomic Science
Ernest Rutherford is renowned for his groundbreaking investigations into radioactivity and the atom. He found that uranium emits alpha and beta rays, two different forms of radiation. He discovered that the atom's mass is centred in its central, positively charged nucleus and that the majority of the atom is made up of space. With the discovery of the nucleus of an atom and the subsequent revelation of the protons, a single atom with a positive charge contained within the nucleus, Ernest Rutherford, thus, forever altered the course of human history.
About Rutherford
Ernest Rutherford was the second son and fourth child of a family of seven sons and five daughters when he was born on August 30, 1871, in Nelson, New Zealand. With Ernest's grandfather and the rest of the family, James Rutherford, a Scottish Ferrier, emigrated to New Zealand in 1842. His mom, née Martha Thompson, an English schoolteacher, moved there in 1855 along with her widowed mother.
Ernest completed his early schooling in public institutions before enrolling at Nelson Collegiate School at the age of 16. He entered Canterbury College at the University of New Zealand in Wellington after receiving a university scholarship in 1889.
Ernest Rutherford theorised the rules of radioactive decay, hypothesised the nuclear nature of the atom, and found alpha and beta rays. In 1908, he was awarded the Chemistry Nobel Prize.

Ernest Rutherford
Ernest Rutherford Discoveries
The Rutherford model of the atom was Rutherford's most significant contribution to science. The teeny building components of all matter are called atoms. For hundreds of years, scientists have been aware of the existence of atoms. But they were unaware of their composition or their mechanisms. In 1911, Rutherford put forth his model. He claimed that most of the atom's mass was contained in the small nucleus and that the rest was space.
Later, as additional research into atoms was conducted, other scientists changed the model. Rutherford's hypothesis, however, marked a fundamental development in our knowledge of atoms. Rutherford also made significant radioactive findings.
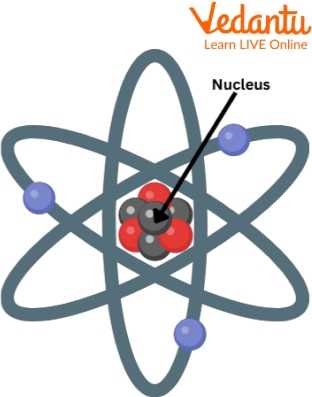
Rutherford’s Structure of the Atom
Ernest Rutherford Atomic Model
Rutherford's atomic model suggests:
An atom's positive charge and the majority of its mass are packed into a very small volume; he was referring to the nucleus of the atom.
According to Rutherford's theory, an atom's nucleus is surrounded by negatively charged electrons. He also stated that the electrons that surround the nucleus travel in a circular pattern at extremely high speeds. He gave these circular routes the name orbits.
A powerful electrical force of attraction holds the negatively charged electrons and the positively charged mass of particles that together make up the nucleus.
Important Points about the Scientist Rutherford
A few important points about Ernest Rutherford.
British physicist Ernest Rutherford (1871–1937), born in New Zealand, won the 1908 Nobel Prize for Chemistry. He is frequently referred to be the "Father of Nuclear Physics,"
Rutherford worked as the chairman of the Physics Department at McGill University after completing his studies with J. J. Thomson at the Cavendish Laboratory at Cambridge University. He carried out the studies that earned him the Nobel Prize at Montreal, where he also distinguished and named alpha, beta, and gamma-rays and discovered the concept of radioactive half-lives.
Rutherford came back to the UK in 1907 to work as a professor at Manchester University. Two years later, in the Geiger-Marsden experiment, he, Hans Geiger, and Ernest Marsden saw alpha particles dispersing backwards when they were fired at a gold foil. It was like if you shot a 15-inch shell at a piece of tissue paper, and it came back and hit you.
According to Rutherford, who described the experiment's unexpected results. Rutherford's model of the atomic nucleus, groundbreaking advancement in nuclear physics, was inspired by these results.
At Cambridge, he was appointed Cavendish Professor of Physics in 1919. In addition, James Chadwick, a colleague and former student of Rutherford, discovered neutrons in 1932. Rutherford also introduced the term "proton" and offered theories regarding their existence.
Summary
From this article, Rutherford Information, we have learnt a lot about the great scientist Rutherford. British scientist Ernest Rutherford was a native of New Zealand. He was a notable pioneer in the field of Nuclear Physics. Rutherford was awarded the Nobel Prize in Chemistry in 1908 for his contributions. In 1892, he received a bachelor's degree from Canterbury. Later, he earned more degrees, one of which was from the English university of Cambridge. Rutherford passed away on October 19, 1937, in Cambridge. At Westminster Abbey, he was laid to rest.
FAQs on Important Points About Scientist Ernest Rutherford
1. Rutherford used gold foil for what purpose?
Rutherford picked gold because it is one of the most malleable metals, and he desired the thinnest coating possible for his scattering experiment.
2. How did Rutherford alter the atom's model?
With his well-known gold foil, which showed that the atom had a small, heavy nucleus, Rutherford overturned Thomson's hypothesis in 1911.
3. What is the rationale behind Rutherford's experiment?
Rutherford reasoned that the mass of an atom was distributed across the atom if Thomson's model was accurate. Then, there wouldn't be much to divert the alpha particles if he fired high-velocity helium nuclei at an atom. He decided to put this to the test using a thin gold coating.









