




Why Are Halogens Important in Everyday Life?
Halogens make one of the most important topics in Chemistry. Now, what are halogens? Let us read Halogen’s definition. Halogens are the six chemical elements in group 17 (VIIA) of the periodic table. They exhibit comparable chemical properties, for example, by producing sodium salts that are highly similar to one another (Na). Therefore, it gets its name from the Greek terms hals, which means "salts," and genes, which means "origin."
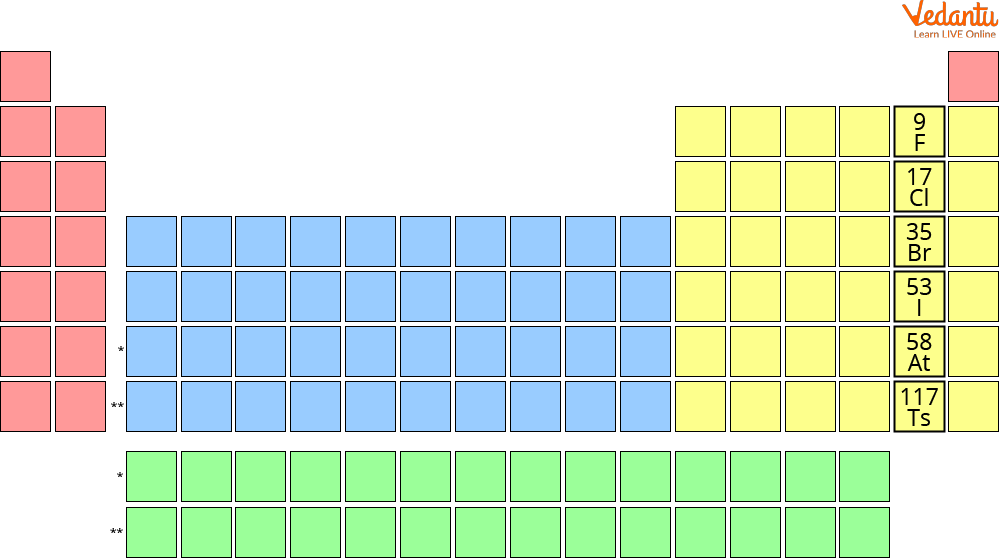
Periodic Table Depicting the Group 17 Halogens
Halogens have been used by humans since ancient times, even before having a deep chemical knowledge that allowed us to distinguish or study them better. They were mainly used in the form of salts, which the ancient Phoenicians and Greeks used as a method of preserving food (brine). Here we will discuss various halogens properties, uses, and examples.
Which Chemical Elements Belong to the Halogen Group?
The halogen group has six elements:
Fluorine (F)
Chlorine (Cl)
Bromine (Br)
Iodine (I)
Astatine (At)
Tennessine (Ts)
Physical Properties of Halogens
The halogens are a diverse group and can be found at room temperature and pressure in all three states of matter:
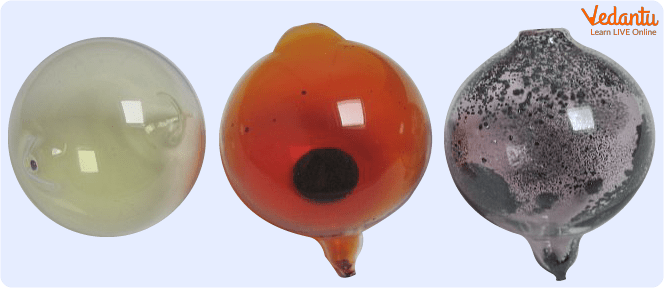
Halogens are Present in all Three States of Matter in Nature
Gassy: Fluorine and Chlorine.
Liquid: Bromine.
Solid: Iodine and astatine.
Regarding colour, they range from pale yellow (fluorine) and yellowish green (chlorine) to brownish red (bromine) and violet or black (iodine). Astatine, on the other hand, is an unstable, radioactive element that does not have a long enough half-life to be seen. On the other hand, Tennessee is a synthetic element whose properties are still being studied.
Chemical Properties of Halogens
Due to their high reactivity, halogens are seldom seen in their monoatomic state but rather as a component of other compounds. They may create diatomic molecules of the same element, at most. F2, Cl2, Br2, and I2, for instance.
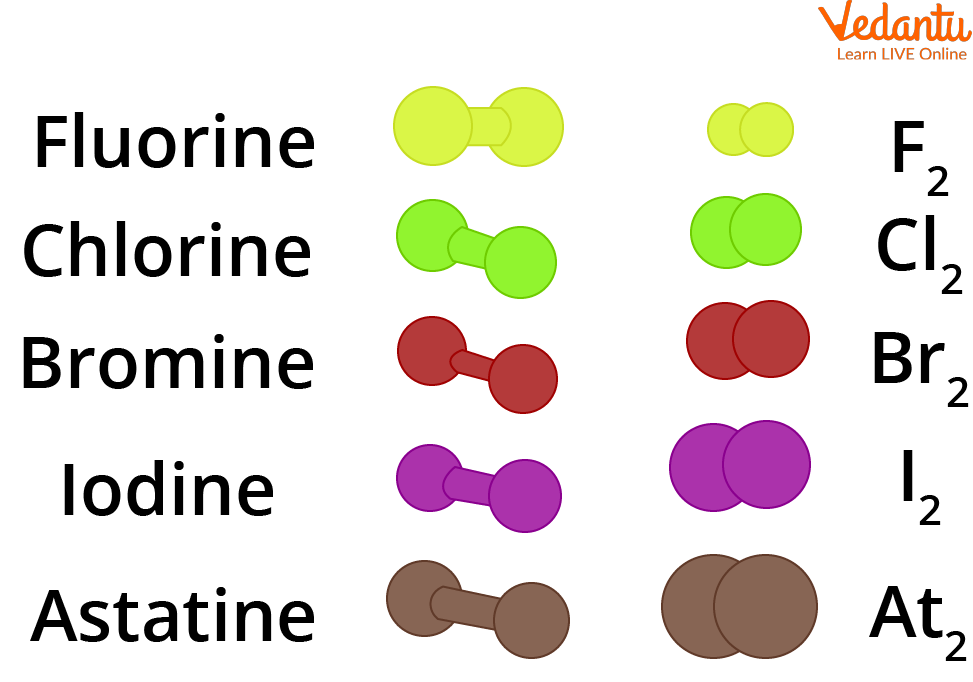
Shapes of Halogen Molecules
Halogens create monovalent ions (-1), meaning they lack the last electron needed to complete their energy level. They are all oxidising substances as a result. On the Pauling scale, its electronegativity is 2.5 as well (or less). The most electronegative element is fluorine. The decreasing order of electronegativity F>Cl>Br>I is what is the order of electronegativity among halogens.
What are Halogens used for?
The uses of halogen elements are numerous. To cure wounds, sanitise surfaces, and safeguard swimming pools and spas, disinfectants like chlorine and bromine are utilised. Both of these substances are frequently employed as flame retardants. Bleach also contains chlorine. Halogen lamps use iodine and bromine to provide a brighter glow than ordinary incandescent lights. In pharmaceuticals, halogens are employed. Nuclear medicine uses the isotopes of astatine and iodine. The photography business uses brominated chemicals (bromides) as a material and as sedatives.
Iodine has antibacterial halogen properties.
In the meanwhile, fluorine is utilised to create Teflon resins, lubricants, and antifreeze.
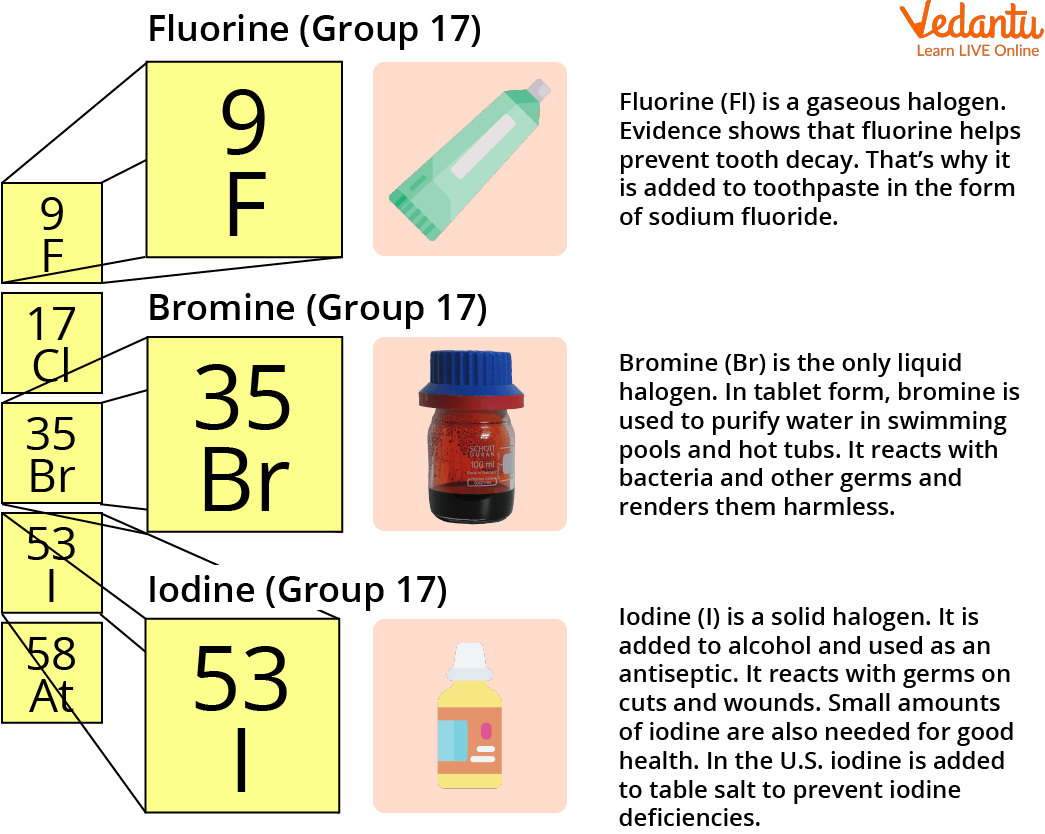
The Uses of Halogen Elements
Examples of Everyday Substances with Halogens
Aerosols once included the halogen-containing chemical CFC.
Some well-known halogen-containing compounds include:
Common salt or table salt (NaCl). It is used to season meals and is edible.
Silver nitrate (AgBr). Because of its photosensitivity, it is employed in photography.
Fluoride in calcium (CaF2). It is utilised in the metallurgical processing of iron and steel.
CFCs, or chlorofluorocarbons. They belong to a group of gases that were historically utilised as aerosol propellants and in refrigeration.
Summary
Halogens are located on the periodic table's right side, just to the left of the noble gas group. Group VII, often known as group 7, or group 17 in more recent IUPAC terminology, designates the halogens. Halogens are all nonmetallic substances. They create brittle solids and have poor heat and electrical conductivity. There is liquid bromine. Astatine and iodine are both solids. Tennessee is thought to be solid by scientists. As a result, the halogen group is the only elemental group that, at ambient temperature and pressure, includes all three of the usual states of matter.
FAQs on Halogens: Key Properties and Real-Life Applications
1. What are the elements in Group 17 of the periodic table, and why are they called 'halogens'?
The elements in Group 17 are Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At). The name 'halogen' originates from Greek words: 'halos' meaning 'salt' and 'genes' meaning 'to produce'. They are called salt-producers because they readily react with metals to form a wide range of salts, such as sodium chloride (NaCl) and potassium bromide (KBr).
2. Are Group 7 and Group 17 the same when referring to the halogens?
Yes, both Group 7 and Group 17 can refer to the halogens. The modern IUPAC convention numbers the groups from 1 to 18, placing the halogens in Group 17. The older system, which is still sometimes used, referred to the main groups using Roman numerals, labelling the halogens as Group VIIA or simply Group 7. For clarity and to align with the current 2025-26 CBSE/NCERT syllabus, Group 17 is the preferred term.
3. What is the general electronic configuration of the halogens?
The general valence shell electronic configuration for the elements in Group 17 (halogens) is ns²np⁵. This means they have seven electrons in their outermost shell. This configuration is just one electron short of the stable noble gas configuration (ns²np⁶), which is the primary reason for their high reactivity, as they have a strong tendency to gain one electron.
4. How do the physical states of halogens change as we move down Group 17?
The physical state of halogens at room temperature changes systematically down the group due to increasing intermolecular forces.
- Fluorine (F₂) and Chlorine (Cl₂) are gases.
- Bromine (Br₂) is a volatile liquid.
- Iodine (I₂) is a solid that sublimes easily.
5. Why does fluorine show anomalous behaviour compared to other halogens?
Fluorine exhibits anomalous properties, differing from other halogens in the group, primarily due to its:
- Small atomic size: This leads to high electronegativity and ionisation enthalpy.
- High electronegativity: It is the most electronegative element, making its chemical bonds unique.
- Absence of d-orbitals: Unlike other halogens, fluorine cannot expand its octet, limiting it to a -1 oxidation state.
- Low F-F bond dissociation enthalpy: Due to repulsion between the lone pairs on the small fluorine atoms, the F-F bond is weaker than expected, contributing to its high reactivity.
6. Why do halogens have a high negative electron gain enthalpy?
Halogens have the most negative electron gain enthalpy in their respective periods. This is because the atoms of these elements have a relatively small size and a high effective nuclear charge. Their valence shell configuration (ns²np⁵) is just one electron away from the stable noble gas configuration. As a result, they have a very strong tendency to accept an additional electron to achieve this stability, releasing a large amount of energy in the process.
7. How does the oxidising power of halogens change down the group?
The oxidising power of halogens decreases down the group from fluorine to iodine. Fluorine is the strongest oxidising agent, while iodine is the weakest. An oxidising agent is a substance that readily accepts electrons. The tendency to accept electrons is highest for fluorine due to its high electronegativity and small size. Therefore, F₂ can oxidise Cl⁻, Br⁻, and I⁻ ions, but I₂ cannot oxidise any of the other halide ions.
8. Are halogens toxic to humans?
Yes, in their elemental form (F₂, Cl₂, Br₂), halogens are highly toxic and corrosive. Inhaling them can cause severe respiratory damage, and direct contact can lead to chemical burns. Chlorine gas, for example, has been used as a chemical weapon. However, in their ionic form (as halide ions like F⁻, Cl⁻), they are essential for life in controlled amounts. For instance, chloride ions are vital for bodily fluids, and fluoride ions are used for dental health.
9. What are interhalogen compounds, and give an example?
Interhalogen compounds are molecules formed when two or more different halogen atoms react with each other. They are generally of the type XX'ₙ, where X is the larger, less electronegative halogen and X' is the smaller, more electronegative halogen. They are often more reactive than the parent halogens (except F₂) because the X-X' bond is weaker than the X-X bond in the parent halogens. A common example is Chlorine trifluoride (ClF₃).
10. Which halogen is the heaviest, and what is unique about it?
The heaviest known halogen is Astatine (At), with an atomic number of 85. What makes Astatine unique is that it is highly radioactive. All of its isotopes are short-lived, with the most stable one having a half-life of just over 8 hours. Due to its radioactivity and scarcity, its chemical properties are not as well-studied as other halogens but are predicted to follow the trends of the group, making it the most metallic of the halogens.









