




Why Is Copper Important? Uses, Properties, and Environmental Impact
Have you ever wondered about the things we use every day, what they are made of, and why they don’t burn if you use them overheat? The utensils that we use daily are made of metals. Metals are sturdy and long-lasting and thus are used in the making of many things like automobiles, cooking utensils, etc.
Metals like aluminium, iron, calcium, and sodium are plentifully available on the Earth's crust. They are available in the vast majority as they do not react with other elements. But today, let us focus on collecting information on copper, copper properties and uses.

Copper - The Main Metal
Introduction to Copper
The word copper has been derived from the Latin word Cuprum, which came from the Latin word for the island of Cyprus, the place where Copper was found. Copper is a metal that is reddish-gold in colour, flexible, malleable, and is a good conductor of heat and electricity.
Copper in the periodic table is in the 11th group. Copper easily combines with other metals to form widely used alloys such as brass and bronze. Copper can be recycled easily, about 70% of the copper which is in use has been recycled at least once.

Copper- Easy to Use
Properties of Copper
The following list presents a few commonly known properties of copper.
Symbol: Cu
The Atomic Number of Copper: 29
Atomic Weight: 63.546
Boiling Point: 2,567 oC
Melting Point: 1,083 oC
Number of Protons: 29
Number of Electrons: 29
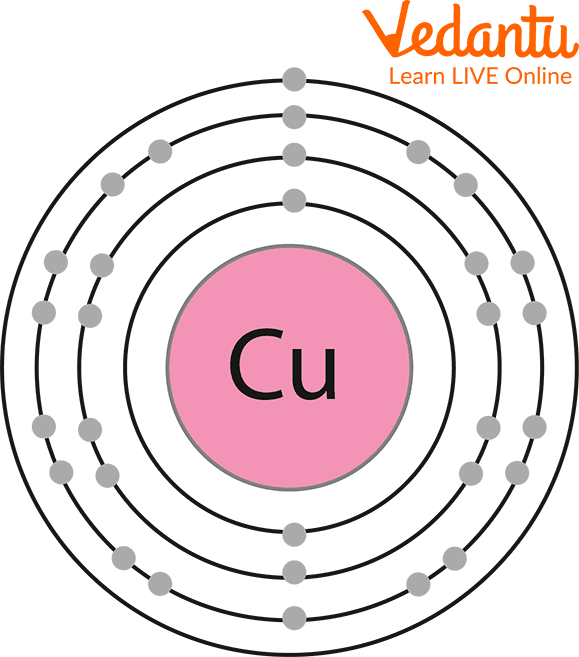
The Shell of Copper
Things Made with Copper
Copper is the most flexible metal with many uses. It often appears in items that we use in our everyday life. Some of the things made out of copper are:
Kitchen Items: The cutlery which is used in your kitchens, most likely contains copper in it. Cookers have been made from Copper for generations. You will come across a lot of antique copper cookers if you visit an older home, in fact, modern cookers also contain copper.
Brass Items: Many items that we use in our everyday routine are made with Brass, which is an alloy made of zinc and copper. All the things that you come across in your house that are made of brass naturally contain copper. The brass lamps that we use at our place contain about 7 pounds of copper, and can you believe how much copper a brass fireplace contains? 10 pounds of copper!
Constructive Materials: Copper is said to be a flexible metal, but it is also a stable material which is why it is used in all sorts of constructive applications. You can also find copper in nails, staples, and power tools, even your air conditioners contain copper in them.
Electronics: Copper is most commonly used in electronic devices. Almost all electric wires, transformers, conductors, and motors use copper because of its efficiency.
Transportation: The transportation industry mostly runs on items made out of copper. Even electric cars roughly contain 55 pounds of copper. Talking commercial aeroplanes, contain about 118 miles of copper wiring.

Things Made with Copper
Interesting Facts About Copper
Now, let’s make facts about copper more fun and engaging. Here are some interesting facts for kids that will fascinate them.
Copper was the first ever metal that was used by man.
In the periodic table, copper can be found as the first element in the eleventh column.
Do you know where you can find copper? Copper is said to be found in the earth’s crust.
Copper is amongst the few metals that are either grey or silver.
Do you know what is used to kill algae and fungi in rivers? It is none other than Copper sulphide.
The largest piece of copper that was ever found was about 520 tons.
Chile is the copper mining capital that produces one-third of copper in the world.
Earth’s Crust
Environmental Effects of Copper
It is a universal fact that a coin has two sides - a positive as well as some negatives. Copper also has some effects that can be coined as hazardous. Let’s see some of them.
The first and foremost is that as copper does not break down in the environment, animals and plants may collect the particles, if and when found in the soil. Plants on soil based on copper have a less chance of survival.
Copper is a severe threat to the farmlands and affects production as well.
Depending upon the acidity that is present in the soil, copper tends to impact the farmlands.
By and large, it is sheep that suffer the most from copper poisoning.
One cannot have copper-based factories near lands that are used for farming.
As copper interferes with soil activity and affects the activities of microorganisms and earthworms.
Summary
In this article, we have learnt about copper properties, uses, atomic number of copper, melting point, boiling point and much more. These properties make copper a perfect material for the making of electrical products as it is a good conductor of electricity. To conclude, we say that copper is an element that has numerous properties, and its uses in daily life cannot be ignored. We hope you enjoyed reading this article; head to our website to read more of such similar articles on metals.
FAQs on Top 10 Amazing Facts About Copper Every Student Should Know
1. What is copper and why is it considered one of the first metals used by humans?
Copper is a chemical element with the symbol Cu, known for its distinct reddish-brown colour. Historians believe it was one of the first metals used by humans because it can be found in a relatively pure form in nature, known as native copper. This meant early civilizations could find and use it without needing complex technology for extraction, marking a significant step up from stone tools.
2. What are the most important properties of copper that make it so useful?
Copper has several unique properties that make it highly valuable for many applications. The key characteristics are:
- Excellent Conductor: It is one of the best conductors of both electricity and heat.
- Ductile: It can be easily stretched into thin wires without breaking.
- Malleable: It can be hammered and shaped into thin sheets.
- Corrosion Resistant: It reacts very slowly with air and water, which makes it very durable.
- Antimicrobial: It naturally kills bacteria and other microbes that come into contact with its surface.
3. Why is copper used for electrical wires instead of a better conductor like silver?
While silver is technically a better conductor of electricity, copper is the standard for electrical wiring due to its excellent balance of conductivity, ductility, and cost. Silver is significantly more expensive, making it impractical for widespread use in homes and infrastructure. Copper provides nearly the same performance as silver but at a much lower price, making it the most economical and efficient choice for electrical applications.
4. How does the Statue of Liberty, which is made of copper, have a green colour?
The Statue of Liberty is covered in copper sheets and was originally the colour of a shiny new coin. Over many years, the copper reacted with oxygen and other chemicals in the air in a process called oxidation. This slow chemical reaction formed a protective green layer called a patina. This patina, a compound known as copper sulfate, now shields the underlying copper from further corrosion.
5. What is the difference between copper, bronze, and brass?
The main difference is that copper is a pure element, while bronze and brass are alloys (mixtures of metals).
- Copper (Cu): A pure, reddish-brown, and relatively soft metal.
- Bronze: An alloy made primarily of copper mixed with tin. Adding tin makes it much harder and more durable than pure copper.
- Brass: An alloy made of copper mixed with zinc. It has a bright, gold-like appearance and is often used for musical instruments and decorative fittings.
6. Where can we find copper being used in our homes and daily lives?
Copper is essential in modern life and is found in many everyday items. Common examples include:
- Electrical Wiring: Inside the walls of our homes and in the power cords for most appliances.
- Pipes and Plumbing: For carrying water, as it resists corrosion and inhibits bacterial growth.
- Cookware: Some high-quality pots and pans have a copper bottom for superior, even heat distribution.
- Electronics: Used in circuit boards for computers, phones, and televisions.
- Coins: Many coins around the world are made from copper alloys.
7. How does storing water in a copper pot help in purifying it?
Copper has natural antimicrobial properties, meaning it can kill or inactivate harmful microorganisms like bacteria and viruses. When water is stored in a copper vessel for a few hours, tiny amounts of copper ions dissolve into the water. These ions are toxic to microbes and disrupt their cellular functions, effectively purifying the water. This is a traditional and scientifically validated method of water purification.









