




Understanding CH2O Molecular Polarity: Structure, Explanation & Examples
Volatile organic compounds (VOCs), which are frequently found in the environment, include carbonyl compounds. Owing to their detrimental impacts on human wellness and plants, and also their crucial functions in photochemical processes, they have drawn more and more attention. One of the carbonyl compounds that the United States Environmental Protection Agency (US EPA) has identified as dangerous pollutants because of its possibility of causing carcinogenicity or respiratory irritation is formaldehyde (CH2O).
One of the more basic naturally occurring aldehydes is formaldehyde. It typically has a gaseous form and a strong, unpleasant odour. This substance can be utilized to create and synthesize a number of different compounds in organizations when utilized in an aqueous form as formalin. Other than the carcinogenic effect CH2O has indeed been utilised to maintain the tissues of the specimens as well as a disinfectant due to its characteristics. Knowing the physical and chemical characteristics of this colourless gas molecule is crucial because it has numerous applications. To understand all of this, one must be familiar with the compound's polarity and molecular structure. So this article explains deeply about CH2O polar or nonpolar nature with its structures.
CH2O Lewis Structure
The core Carbon atom in the CH2O Lewis structure has single bonds with 2 hydrogen atoms and a double bond with an oxygen atom. The core atom does not have any lone pairs of electrons, whereas the oxygen atom does have two. We can easily establish the compound's molecular structure since it also possesses an sp2 hybridization.
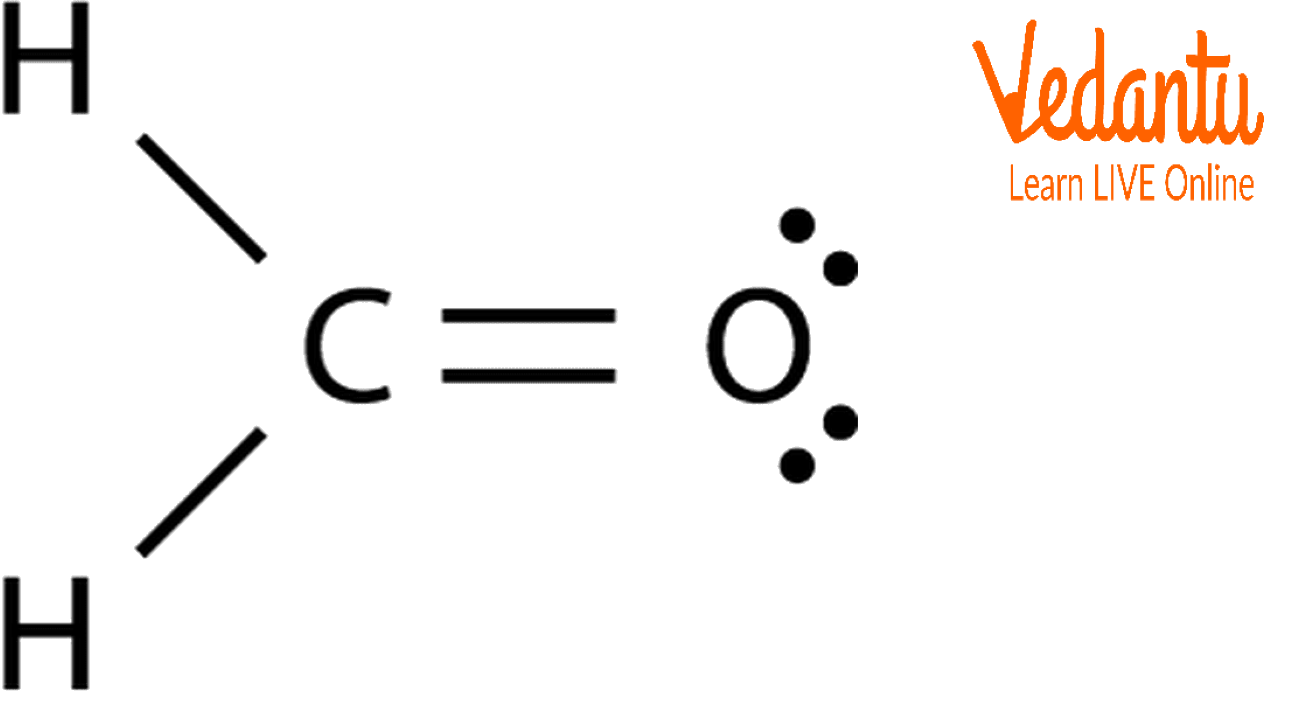
Lewis Structure of CH2O
Molecular Geometry of CH2O
The oxygen atom's non-bonding pair of electrons is dispersed uniformly to lessen the attractive forces among these lone pairs of electrons in CH2O. The CH2O carbon atom has a steric number of three because there are three electron zones surrounding the main atom. Additionally, CH2O possesses AB3 formula and sp2 hybridization, as per valence shell electron pair repulsion (VSEPR) theory.
Bond Angle of CH2O
The valence shell electron pair repulsion (VSEPR) theory states that in order to prevent repulsive forces, the electron clouds must be as wide apart as feasible. Additionally, because there are zero lone pairs of electrons on the central atom of CH2O, the bound pair of electrons is equally distributed, and each atom has a 120-degree bond angle with the central atom of CH2O.
Atoms are located in the sides of the triangle, and carbon is in the middle of the plane created by the three electron clouds.
A trigonal planar shape is created as a consequence of this configuration, molecular geometry, and bond angles. In light of this, sp2 hybridization gives CH2O a trigonal planar form.
Is CH2O Polar or Nonpolar?
If a compound has an uneven distribution of charges, it is said to be polar. A compound is referred to as non-polar if the charge distribution becomes evenly distributed over different regions of the molecule.
CH2O is a polar molecule. It is composed of 3 distinct atoms: a central carbon (C) atom is joined by single and double covalent bonds to two hydrogens (H) atoms and an oxygen (O) atom, accordingly.
In a C-H bond, there exists a slight distinction in the electro-negativity of the C and H atoms. A C=O bond's O and C atoms, although, possess very large differences of electro-negativities from one another. Partially negative charges are created on the oxygen atom due to this difference in electro-negativities, whereas partially positive charges are created on the carbon and hydrogen atoms.
As a result, there is a mismatch in the charges within the compound, which gives rise to the dipole moment connecting the atoms and causes the CH2O molecule to be polar. Oxygen seeks to pull the bound pair of electrons to its end since it is more electronegative than other elements, which raises the negative electrical charge on the oxygen atom.
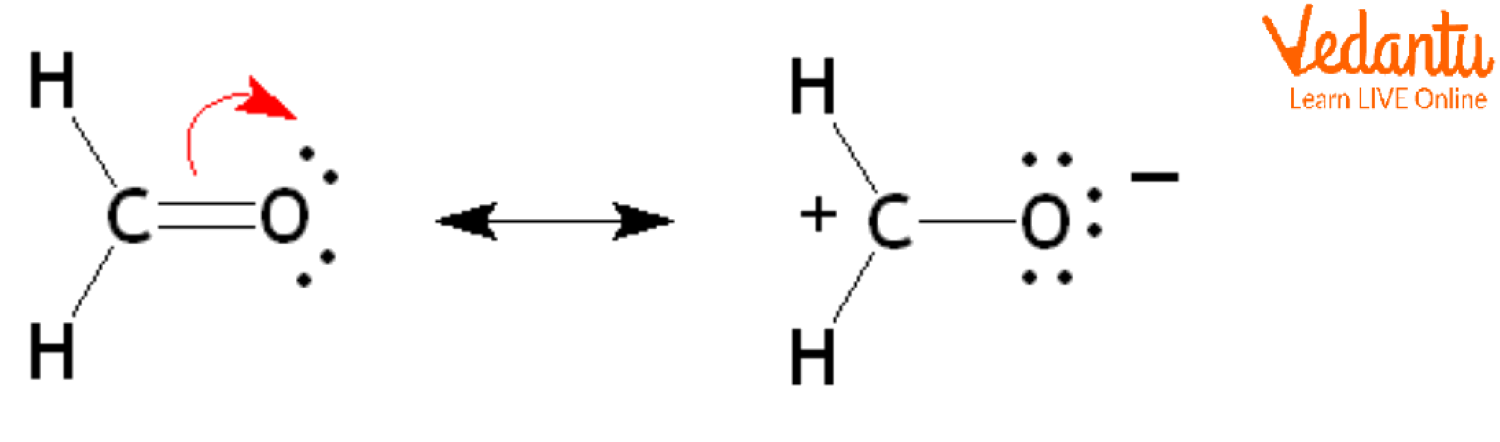
Dipole Moment of CH2O
Key Features to Remember
CH2O is a chemical compound that is colourless, pungent, and rapidly ignites.
The molecular geometry of CH2O is AB3, and it has a trigonal planar structure with sp2 hybridization.
The bond angles are 120 degrees, giving CH2O a trigonal planar geometry.
Because of the disparity in partial charges on the carbon and oxygen atoms, hence CH2O is polar in nature
The core atom of formaldehyde does not have any lone pairs of electrons, while the oxygen atom does have two lone pairs of electrons.
Key Features to Remember
CH2O is a chemical compound that is colourless, pungent, and rapidly ignites.
The molecular geometry of CH2O is AB3, and it has a trigonal planar structure with sp2 hybridization.
The bond angles are 120 degrees, giving CH2O a trigonal planar geometry.
Because of the disparity in partial charges on the carbon and oxygen atoms, hence CH2O is polar in nature
The core atom of formaldehyde does not have any lone pairs of electrons, while the oxygen atom does have two lone pairs of electrons.
List of Related Articles
FAQs on Is CH2O Polar or Nonpolar?
1. Is formaldehyde (CH2O) a polar or nonpolar molecule?
Formaldehyde (CH2O) is a polar molecule. This is because of the significant difference in electronegativity between the carbon (2.55) and oxygen (3.44) atoms, which creates a highly polar carbon-oxygen double bond. Although the molecule has a symmetrical trigonal planar shape, the unequal distribution of electron density results in a net dipole moment, making the overall molecule polar.
2. What is the molecular geometry and shape of CH2O?
The molecular geometry of formaldehyde (CH2O) is trigonal planar. According to VSEPR theory, the central carbon atom is bonded to three other atoms (two hydrogen, one oxygen) and has no lone pairs of electrons. This arrangement causes the atoms to lie in the same plane with bond angles of approximately 120 degrees.
3. What types of chemical bonds are present in a CH2O molecule?
A formaldehyde molecule contains several types of covalent bonds:
- Two polar covalent C-H single bonds.
- One highly polar covalent C=O double bond, which itself is composed of one sigma (σ) bond and one pi (π) bond.
4. What is the hybridization of the central carbon atom in formaldehyde?
The central carbon atom in formaldehyde (CH2O) is sp² hybridized. It uses its three sp² hybrid orbitals to form three sigma (σ) bonds: one with the oxygen atom and two with the hydrogen atoms. The unhybridized p-orbital on the carbon atom overlaps with a p-orbital from the oxygen atom to form the pi (π) bond in the C=O double bond.
5. Why is CH2O considered polar even though its molecular shape is symmetrical?
While the trigonal planar geometry of CH2O is structurally symmetrical, the molecule is electronically asymmetrical. The terminal atoms attached to the central carbon are not identical (one oxygen and two hydrogens). Oxygen is much more electronegative than hydrogen, so it pulls electron density much more strongly. This imbalance means the individual bond dipoles do not cancel each other out, resulting in a net molecular dipole moment and making the molecule polar.
6. How does the Lewis structure for CH2O help in determining its polarity?
The Lewis structure for CH2O shows a central carbon atom, two hydrogen atoms, and an oxygen atom with two lone pairs. It clearly illustrates the C=O double bond. This structure is crucial because it highlights the large electronegativity difference between carbon and oxygen. This difference creates a strong bond dipole pointing towards the oxygen atom, which is not cancelled by the weaker C-H bond dipoles, thus confirming the molecule's overall polarity.
7. What causes the net dipole moment in the CH2O molecule?
The net dipole moment in CH2O arises from the vector sum of its individual bond dipoles. The C=O bond has a very large dipole moment pointing towards the oxygen atom. The two C-H bonds have much smaller dipoles pointing towards the carbon atom. Because these vectors are arranged in a trigonal planar geometry and are unequal in magnitude, they do not cancel out. The dominant C=O dipole creates a strong, non-zero net dipole moment for the entire molecule, with a value of approximately 2.33 D.
8. How does the polarity of CH2O affect its physical properties like boiling point and solubility?
The polarity of CH2O directly influences its physical properties due to the presence of strong intermolecular forces. Its effects include:
- Boiling Point: As a polar molecule, CH2O experiences strong dipole-dipole interactions, which require more energy to overcome than the London dispersion forces in nonpolar molecules of similar size. This gives it a relatively high boiling point (-19 °C).
- Solubility: Following the principle of "like dissolves like," the polarity of formaldehyde makes it soluble in water and other polar solvents.
9. What are the main intermolecular forces (IMF) in formaldehyde?
The main intermolecular forces present in a sample of formaldehyde (CH2O) are:
- Dipole-dipole interactions: These are the most significant IMF in CH2O, occurring because of the attraction between the partially positive end of one polar molecule and the partially negative end of another.
- London dispersion forces: These weaker forces are also present, as they exist in all molecules due to temporary electron cloud fluctuations.
Formaldehyde does not exhibit hydrogen bonding as it does not have a hydrogen atom directly bonded to an oxygen, nitrogen, or fluorine atom.
























