Respiration in Organisms Class 7 important questions with answers PDF download
FAQs on Important Questions For Class 7 Science Chapter 6 Respiration in Organisms - 2025-26
1. What is the Difference Between Respiration and Breathing?
Breathing is the process of inhaling and exhaling air by the organs responsible for gaseous exchange. Respiration is the entire process of gaseous exchange occurring at the cellular level. Check the answers to the Class 7 Science Ch 6 important questions to find more about the differences between respiration and breathing.
2. Why Should You Prefer Vedantu for NCERT Solutions for Class 7 Science Chapter 6 Important Questions?
Class 7 students prefer Vedantu for the important questions of all the chapters Science for the quality explanation of the conceptual answers. They can learn this chapter of Class 7 in a better way by referring to the important questions provided.
3. What do you mean by respiration?
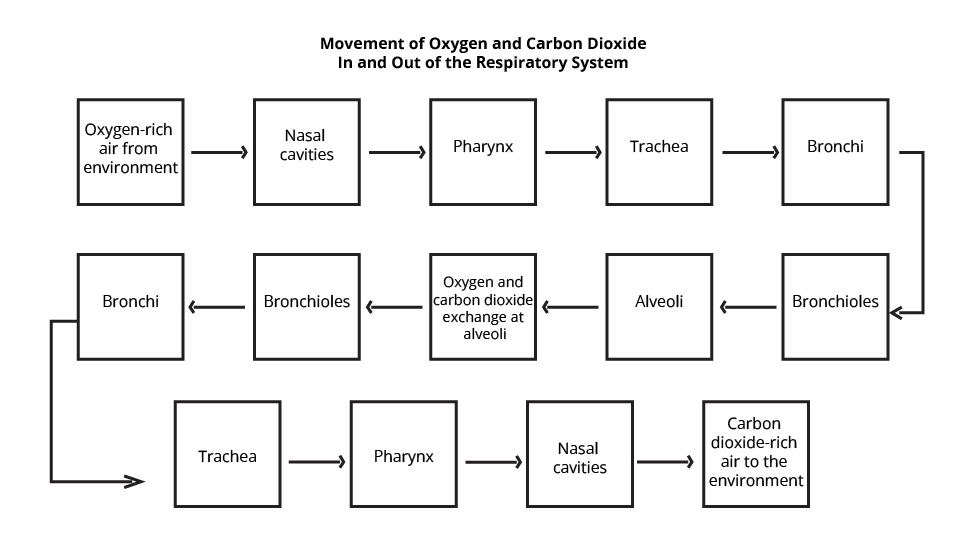
Respiration is a biological process in which stored food is oxidized in the presence of oxygen and carbon dioxide is released from the system to supply energy to the body's cells and allow them to carry out their tasks. During respiration, an organism provides its cells and tissues with oxygen for metabolism and removes carbon dioxide produced during energy-producing activities. This is accomplished by physical and chemical mechanisms (such as breathing and diffusion).
4. What do you mean by breathing?
Breathing is the process by which oxygen-rich air is absorbed into an organism's body and carbon dioxide-rich air is evacuated (with the aid of breathing organs). During breathing, both inhalation and exhalation occur on a regular basis. A breath is defined as one intake followed by one exhale. Inhaling, or inspiration is the initial process. The diaphragm undergoes contraction and pulls downward during the process of inhalation. The muscles that are present between the ribs tend to tighten and pull upward at the same instant.
5. How do I download the Important questions for Class 7 Science Chapter 6?
The important questions are easily available on the Vedantu site.
Click on this link-Important Questions for Class 7 science Chapter 6.
The webpage with Vedantu’s solutions for Class 7 Science Chapter 6 will open.
To download this, click on the Download PDF button and you can view the solutions offline.
All the solutions and their PDF file are free to download on the Vedantu site. You do not require to pay anything in order to avail them.
6. What is the importance of respiration?
Respiration is vital since it generates energy that is required for the body's regular functioning. Respiration delivers oxygen to cells while also removing harmful carbon dioxide. Heat is also produced as part of the energy released by breathing. To provide the body's cells the energy they need to function, stored food is oxidized in the presence of oxygen and carbon dioxide is expelled from the system. It is a very crucial phenomenon of the human body and keeps the body’s energy transfer cycle in check. For more information, you can visit Vedantu app.
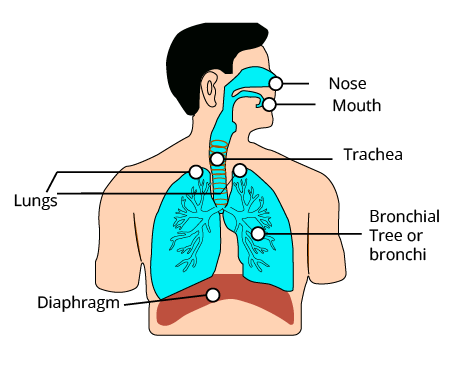
7. Why is the importance of our lungs?
Your lungs are responsible for bringing new oxygen into your body. They eliminate carbon dioxide and other waste gases that your body does not require. The muscles of the rib cage, particularly the main muscle, the diaphragm, are used to breathe in (inhale). Your lungs deliver oxygen and remove carbon dioxide from your body, allowing every other organ to operate properly. Respiratory issues can be caused by genetics, illness, and the environment.