Science Notes for Chapter 6 Reproduction in Animals Class 8 - FREE PDF Download
FAQs on CBSE Notes Class 8 Science Chapter 6 - Reproduction in Animals - 2025-26
1. What are the main topics to revise in Class 8 Science Chapter 6, Reproduction in Animals?
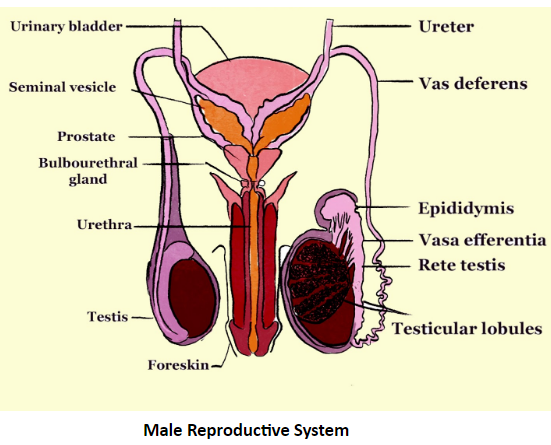
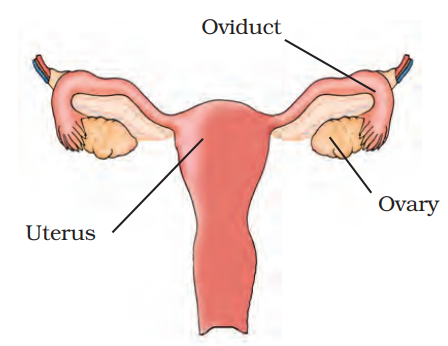
For a quick revision of this chapter, you should focus on the core concepts. These include the two primary modes of reproduction, sexual and asexual, the male and female reproductive organs, the process of fertilisation and subsequent development of the zygote into an embryo, the distinction between viviparous and oviparous animals, and the concept of metamorphosis as seen in frogs.
2. How can you quickly summarise the two main modes of reproduction in animals?
The two modes of reproduction can be summarised as follows:
- Sexual Reproduction: This process involves two parents, a male and a female. It requires the fusion of their respective gametes (sperm and egg) to form a zygote, resulting in offspring with genetic traits from both parents.
- Asexual Reproduction: This process involves only a single parent. The offspring produced are genetically identical to the parent, as there is no fusion of gametes. Examples include budding and binary fission.
3. Why do offspring from sexual reproduction show variation, while those from asexual reproduction are identical to the parent?
Offspring from sexual reproduction show variation because they inherit a unique combination of genes from two different parents through the fusion of sperm and egg. In contrast, asexual reproduction involves only one parent passing on its exact genetic material to the offspring, resulting in a genetically identical clone.
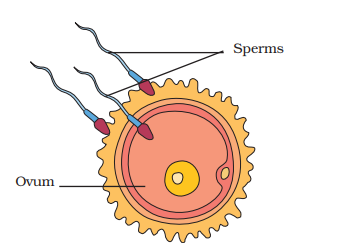
4. What is the correct sequence of development from fertilisation to foetus in humans?
The development follows a specific sequence. First, the fusion of a sperm and an ovum, called fertilisation, results in the formation of a single cell known as a zygote. The zygote undergoes repeated cell division to form an embryo, which then embeds in the uterus wall. The embryo continues to develop until its body parts are identifiable, at which stage it is called a foetus.
5. For revision, what is the main difference between internal and external fertilisation?
The key difference lies in the location where the fusion of gametes occurs. Internal fertilisation takes place inside the female's body, as seen in humans, cows, and hens. External fertilisation happens outside the female's body, typically in an aquatic environment, as seen in frogs and fish where eggs and sperm are released into the water.
6. How do viviparous and oviparous animals differ in their reproductive strategy?
The primary difference is in how they bring forth their young. Viviparous animals, such as humans and dogs, give birth to live young that have developed inside the mother's body. In contrast, oviparous animals, like birds and reptiles, lay eggs. The embryo develops inside the egg, drawing nourishment from the yolk, and hatches outside the mother's body.
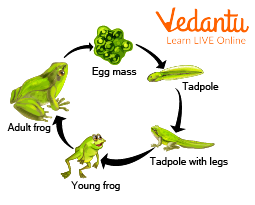
7. What does 'metamorphosis' mean in the life cycle of a frog, and why is this concept important?
Metamorphosis refers to the drastic transformation an animal undergoes from its larval stage to its adult form. In a frog's life cycle, the aquatic larva, known as a tadpole, changes into an adult frog. This concept is important because it highlights that the young of some animals can look completely different from the adults, unlike in humans where babies are born with the same basic body structure as adults.
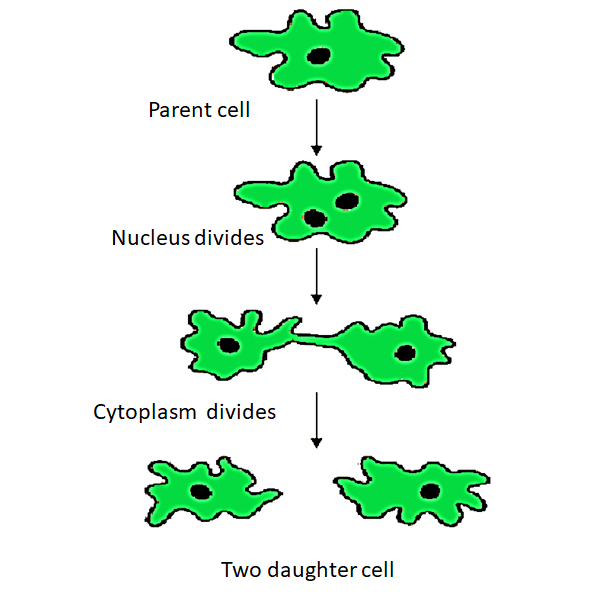
8. How can you distinguish between budding and binary fission for your exam revision?
The main distinguishing factor is the method of division. In budding, a new organism develops from an outgrowth or 'bud' on the parent's body, as seen in Hydra. In binary fission, a single-celled organism, like an Amoeba, splits into two equal, identical daughter cells. Budding involves an outgrowth, while binary fission is a complete split of the parent organism.