
What is Zeise’s salt and ferrocene? Explain with structure?
Answer
595.8k+ views
Hint: The chemical formula of Zeise’s salt is ${\rm{K}}\left[ {{\rm{PtC}}{{\rm{l}}_{\rm{3}}}\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \right)} \right]{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{O}}$. Ferrocene is an organometallic compound.
Complete step-by-step answer:
Zeise’s salt is an organometallic compound having the chemical formula ${\rm{K}}\left[ {{\rm{PtC}}{{\rm{l}}_{\rm{3}}}\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \right)} \right]{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{O}}$, that is, potassium trichloro(ethylene)platinate (II).
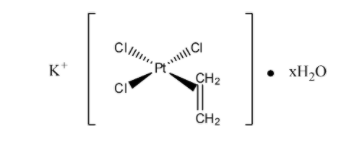
A. Zeise’s salt has a square planar geometry. ${\rm{K}}\left[ {{\rm{PtC}}{{\rm{l}}_{\rm{3}}}\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \right)} \right]{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{O}}$ is monohydrated. The ${\rm{\sigma }}$-bond in Zeise’s salt is not localized between the carbon and the metal. The bonding in this salt can be possible to explain using molecular orbital approach. It can summarize as follows:
1) In olefin, the ${\rm{\pi }}$-electron density overlaps with the orbital of the metal having ${\rm{\sigma }}$-type acceptor.
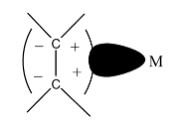
Donation from the filled ${\rm{\pi }}$-orbitals to vacant metal orbitals
2) One back bonding results from the flow of electron density from a filled metal ${\rm{d\pi }}$orbital into the vacant antibonding orbitals of the C-atom.
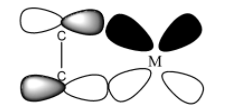
Back bonding taking place from the filled metal orbital to accept ${{\rm{\pi }}^{\rm{*}}}$orbitals
B. Ferrocene is a organometallic compound having the chemical formula ${{\rm{C}}_{{\rm{10}}}}{{\rm{H}}_{{\rm{10}}}}{\rm{Fe}}$. Ferrocene is an orange-yellow compound. The odour of ferrocene is like that of camphor. Ferrocene is soluble in benzene solution, but not soluble in water. Ferrocene has the chemical formula ${\rm{Fe}}{\left( {{{\rm{\eta }}^{\rm{5}}} - {{\rm{C}}_{\rm{5}}}{{\rm{H}}_{\rm{5}}}} \right)_{\rm{2}}}$. In ferrocene, the ${\rm{Fe}}$ is placed or sandwiched between the two rings of cyclopentadienyl rings usually in the staggered conformation. In the lowest energy state, the ferrocene has an eclipsed conformation.
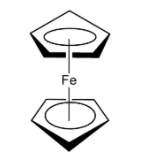
Note: Students may get confused between the structure of Zeise’s salt and ferrocene. Zeise’s salt has the chemical formula ${\rm{K}}\left[ {{\rm{PtC}}{{\rm{l}}_{\rm{3}}}\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \right)} \right]{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{O}}$. In Zeise’s salt, the ${\rm{Pt}}\left( {{\rm{II}}} \right)$consists of three ${\rm{\pi }}$-type filled d-orbital which are ${{\rm{d}}_{{\rm{xy}}}}{\rm{,}}{{\rm{d}}_{{\rm{yz}}}}$ and ${{\rm{d}}_{{\rm{xz}}}}$. Ferrocene is an organometallic compound and has ${{\rm{C}}_{\rm{5}}}$ rotational axis and contains five perpendicular ${{\rm{C}}_2}$ axis. Ferrocene has a point group ${{\rm{D}}_{\rm{5}}}$.
Complete step-by-step answer:
Zeise’s salt is an organometallic compound having the chemical formula ${\rm{K}}\left[ {{\rm{PtC}}{{\rm{l}}_{\rm{3}}}\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \right)} \right]{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{O}}$, that is, potassium trichloro(ethylene)platinate (II).

A. Zeise’s salt has a square planar geometry. ${\rm{K}}\left[ {{\rm{PtC}}{{\rm{l}}_{\rm{3}}}\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \right)} \right]{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{O}}$ is monohydrated. The ${\rm{\sigma }}$-bond in Zeise’s salt is not localized between the carbon and the metal. The bonding in this salt can be possible to explain using molecular orbital approach. It can summarize as follows:
1) In olefin, the ${\rm{\pi }}$-electron density overlaps with the orbital of the metal having ${\rm{\sigma }}$-type acceptor.

Donation from the filled ${\rm{\pi }}$-orbitals to vacant metal orbitals
2) One back bonding results from the flow of electron density from a filled metal ${\rm{d\pi }}$orbital into the vacant antibonding orbitals of the C-atom.

Back bonding taking place from the filled metal orbital to accept ${{\rm{\pi }}^{\rm{*}}}$orbitals
B. Ferrocene is a organometallic compound having the chemical formula ${{\rm{C}}_{{\rm{10}}}}{{\rm{H}}_{{\rm{10}}}}{\rm{Fe}}$. Ferrocene is an orange-yellow compound. The odour of ferrocene is like that of camphor. Ferrocene is soluble in benzene solution, but not soluble in water. Ferrocene has the chemical formula ${\rm{Fe}}{\left( {{{\rm{\eta }}^{\rm{5}}} - {{\rm{C}}_{\rm{5}}}{{\rm{H}}_{\rm{5}}}} \right)_{\rm{2}}}$. In ferrocene, the ${\rm{Fe}}$ is placed or sandwiched between the two rings of cyclopentadienyl rings usually in the staggered conformation. In the lowest energy state, the ferrocene has an eclipsed conformation.

Note: Students may get confused between the structure of Zeise’s salt and ferrocene. Zeise’s salt has the chemical formula ${\rm{K}}\left[ {{\rm{PtC}}{{\rm{l}}_{\rm{3}}}\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \right)} \right]{\rm{.}}{{\rm{H}}_{\rm{2}}}{\rm{O}}$. In Zeise’s salt, the ${\rm{Pt}}\left( {{\rm{II}}} \right)$consists of three ${\rm{\pi }}$-type filled d-orbital which are ${{\rm{d}}_{{\rm{xy}}}}{\rm{,}}{{\rm{d}}_{{\rm{yz}}}}$ and ${{\rm{d}}_{{\rm{xz}}}}$. Ferrocene is an organometallic compound and has ${{\rm{C}}_{\rm{5}}}$ rotational axis and contains five perpendicular ${{\rm{C}}_2}$ axis. Ferrocene has a point group ${{\rm{D}}_{\rm{5}}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




