
What is Z in the following reaction sequence?
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\xrightarrow[(ii){{H}_{3}}P{{O}_{2}}+{{H}_{2}}O(iii)CO;anhydrousAlC{{l}_{3}}/CuCl]{(i)NaN{{O}_{2}}/HCl,273K}Z\]
A)${{C}_{6}}{{H}_{5}}C{{O}_{2}}H$
B)${{C}_{6}}{{H}_{5}}OH$
C)${{C}_{6}}{{H}_{5}}CHO$
D)${{C}_{6}}{{H}_{6}}$
Answer
569.4k+ views
Hint: The answer to this question lies in the basic concepts of organic chemistry which includes the fact that sodium nitrite yields diazonium salt and undergoes reduction when treated with hypophosphorous acid followed by subsequent formylation.
Complete answer:
In our organic chemistry classes, we have studied about the basic reactions that involve several named reactions as well as oxidation, reduction, substitution etc., reactions.
Let us now see the types of reaction aniline undergoes when subjected to subsequent reagents one by one.
- Aniline when treated with sodium nitrite in presence of hydrochloric acid in cold condition yields the diazonium salt because there is nitration occurring.
Further this diazonium salt produced is subjected to the treatment with hypophosphorous acid that is ${{H}_{3}}P{{O}_{2}}$ which is basically a reducing agent, reduces the salt with the elimination of amino groups and thus addition of hydrogen occurs by hydrolysis.
The product obtained is benzene and this is further treated with the carbon monoxide in the presence of aluminium chloride and copper chloride converts it into benzaldehyde. This reaction is called Gatterman Koch reaction used for formylation of aromatic compounds.
The overall reaction is as shown below,
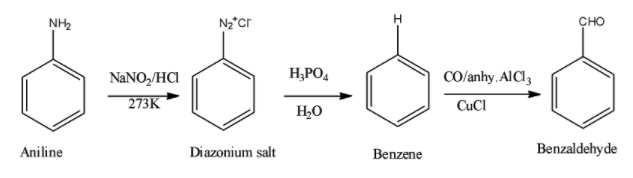
Thus, the correct answer is option C) ${{C}_{6}}{{H}_{5}}CHO$
Note: The main point to be noted here is that nitration doesn’t always means reaction of sulphuric and nitric acid but it is the addition of nitronium ion and is valid to any reagent which are capable to produce this ion based on the reaction conditions and also type of reactant used.
Complete answer:
In our organic chemistry classes, we have studied about the basic reactions that involve several named reactions as well as oxidation, reduction, substitution etc., reactions.
Let us now see the types of reaction aniline undergoes when subjected to subsequent reagents one by one.
- Aniline when treated with sodium nitrite in presence of hydrochloric acid in cold condition yields the diazonium salt because there is nitration occurring.
Further this diazonium salt produced is subjected to the treatment with hypophosphorous acid that is ${{H}_{3}}P{{O}_{2}}$ which is basically a reducing agent, reduces the salt with the elimination of amino groups and thus addition of hydrogen occurs by hydrolysis.
The product obtained is benzene and this is further treated with the carbon monoxide in the presence of aluminium chloride and copper chloride converts it into benzaldehyde. This reaction is called Gatterman Koch reaction used for formylation of aromatic compounds.
The overall reaction is as shown below,

Thus, the correct answer is option C) ${{C}_{6}}{{H}_{5}}CHO$
Note: The main point to be noted here is that nitration doesn’t always means reaction of sulphuric and nitric acid but it is the addition of nitronium ion and is valid to any reagent which are capable to produce this ion based on the reaction conditions and also type of reactant used.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




