
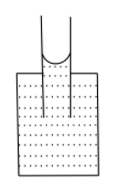
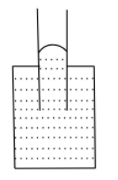
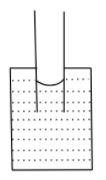
You dip a capillary tube into a beaker of different fluids, and from observation of the shape of the fluid surface deduce that in which figure, cohesive forces dominate over adhesive forces:
A.
B.
C.
D.
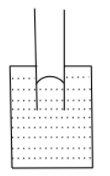




Answer
565.5k+ views
Hint: When the molecules of the same substance attract each other, this force is known as the cohesive force. When the molecules of different substances (in contact) attract each other, this force is known as adhesive force.
Complete step by step answer:
When a fluid is put into a container (say a liquid) then the molecules in the bulk experience only cohesive forces but the molecules at the boundary experience adhesive forces as well due to direct contact with the container.
The cohesive forces tend to keep the molecules of the fluid together, on the other hand, the adhesive forces tend to increase the surface contact between the container and the liquid.
Thus when the liquid is kept inside a capillary tube, the surface of contact is very large, thus the adhesive and cohesive forces come into play. And whichever force dominates, decides if the liquid should rise or fall in a capillary tube, it also decides if a concave or convex meniscus is formed.
If the adhesive force dominates, the attraction between the surface of the container and liquid molecules is more, and the liquid rises in the capillary tube, it also forms a concave shape.
If the cohesive force dominates, the liquid molecules are more attracted to each other and the liquid wants to have the least surface contact with the container, thus liquid falls in the capillary tube and forms a convex shape.
Hence, the correct answer is option (D).
Note: The adhesive and cohesive forces are collective terms referring to the intermolecular forces of attraction between molecules of a liquid or between liquid and a surface. The cohesive forces arise due to van der Waals force or Hydrogen bonding, whereas the adhesive forces may arise due to mechanical forces or electrostatic forces.
Complete step by step answer:
When a fluid is put into a container (say a liquid) then the molecules in the bulk experience only cohesive forces but the molecules at the boundary experience adhesive forces as well due to direct contact with the container.
The cohesive forces tend to keep the molecules of the fluid together, on the other hand, the adhesive forces tend to increase the surface contact between the container and the liquid.
Thus when the liquid is kept inside a capillary tube, the surface of contact is very large, thus the adhesive and cohesive forces come into play. And whichever force dominates, decides if the liquid should rise or fall in a capillary tube, it also decides if a concave or convex meniscus is formed.
If the adhesive force dominates, the attraction between the surface of the container and liquid molecules is more, and the liquid rises in the capillary tube, it also forms a concave shape.
If the cohesive force dominates, the liquid molecules are more attracted to each other and the liquid wants to have the least surface contact with the container, thus liquid falls in the capillary tube and forms a convex shape.
Hence, the correct answer is option (D).
Note: The adhesive and cohesive forces are collective terms referring to the intermolecular forces of attraction between molecules of a liquid or between liquid and a surface. The cohesive forces arise due to van der Waals force or Hydrogen bonding, whereas the adhesive forces may arise due to mechanical forces or electrostatic forces.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




