
How do you write/draw the Lewis structure for N?
Answer
572.7k+ views
Hint Lewis dot structures also known as electron dot structures. Lewis dot structures represent the valence electrons of the atoms present within the molecule. By Lewis dot structures we can visualize the bonding electrons and lone pair of electrons present in the molecule.
Complete step by step answer:
- In the question it is asked to draw the Lewis structure for N (nitrogen atom).
- Nitrogen belongs to group 15 and is present in the p-block of the periodic table.
- The atomic number of nitrogen is 7.
- Means nitrogen has 7 electrons in its electronic configuration.
- The electronic configuration of the nitrogen atom is $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$ .
- From the above electronic configuration we can say that the nitrogen has 5 five electrons in 2s and 2p orbitals and the number of valence electrons are five in nitrogen.
- The Lewis dot structure of nitrogen atom is as follows.
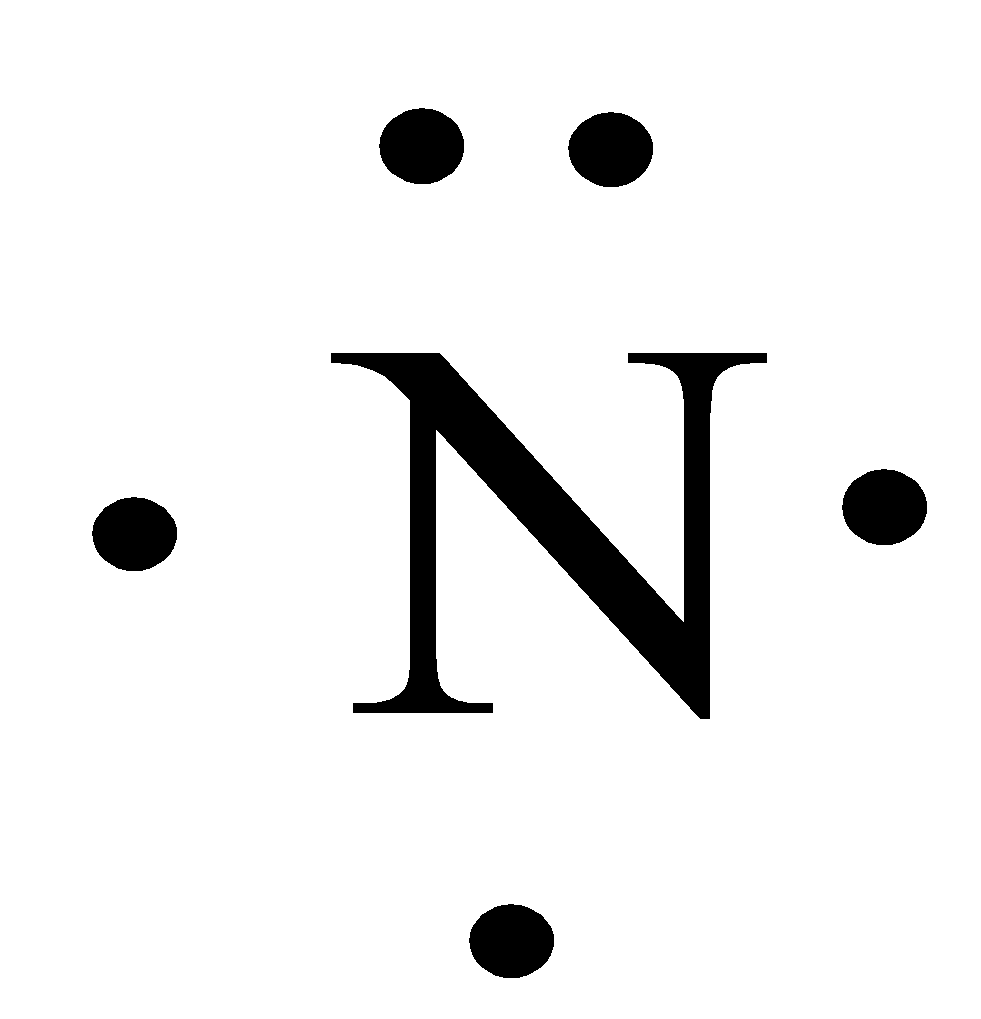
- From the above Lewis dot structure of nitrogen we can say that nitrogen has only one lone pair of electrons and three single electrons around the nitrogen atom.
Note: Without knowing the electronic configuration of the atom we cannot draw the Lewis dot structure of the atoms or elements. The three single electrons present around the nitrogen makes the nitrogen atom to form three bonds with other atoms. In all the compounds which have nitrogen atoms in its composition, nitrogen will have three bonds with other atoms.
Complete step by step answer:
- In the question it is asked to draw the Lewis structure for N (nitrogen atom).
- Nitrogen belongs to group 15 and is present in the p-block of the periodic table.
- The atomic number of nitrogen is 7.
- Means nitrogen has 7 electrons in its electronic configuration.
- The electronic configuration of the nitrogen atom is $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$ .
- From the above electronic configuration we can say that the nitrogen has 5 five electrons in 2s and 2p orbitals and the number of valence electrons are five in nitrogen.
- The Lewis dot structure of nitrogen atom is as follows.

- From the above Lewis dot structure of nitrogen we can say that nitrogen has only one lone pair of electrons and three single electrons around the nitrogen atom.
Note: Without knowing the electronic configuration of the atom we cannot draw the Lewis dot structure of the atoms or elements. The three single electrons present around the nitrogen makes the nitrogen atom to form three bonds with other atoms. In all the compounds which have nitrogen atoms in its composition, nitrogen will have three bonds with other atoms.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




