
Write the structures of the major products expected from the following reactions:
A.Mononitration of 3-methylphenol
B.Dinitration of 3-methylphenol
C.Mononitration of phenyl methanoate
Answer
573.6k+ views
Hint: We have to know that the nitration of aromatic compounds is electrophilic aromatic substitution. The product formed would depend on the incoming substitution. If the substituent is ortho-para director, then ortho-para major products would be dominated and meta product would be minor byproduct. If the substituent is a meta director, then the product formed would dominate in the meta position.
Complete answer:
We know that in case of substituted aromatic compounds, the functional group present in the compound directs the next incoming group to a specific position in the aromatic ring. We call this as the directive influence of the group already bonded to the benzene ring.
If a monosubstituted compound is treated with an electrophile, it will undergo electrophilic aromatic substitution reaction and form a disubstituted compound which may be recognized using the descriptors ortho, meta, and para. If the relative yield of the ortho product and the para products are greater than that of the meta product, then the substituent found in the monosubstituted ring is named an ortho, para directing group.
We know that the methyl group and hydroxyl present in the benzene ring is ortho and para directing. Since the methyl group activates the ring at the para and ortho position much more than the meta positions, the electrophilic substitution reactions occur at these positions.
The influence of hydroxyl group and methyl group decides the incoming group position.
The major products formed by the mononitration of 3-methylphenol.
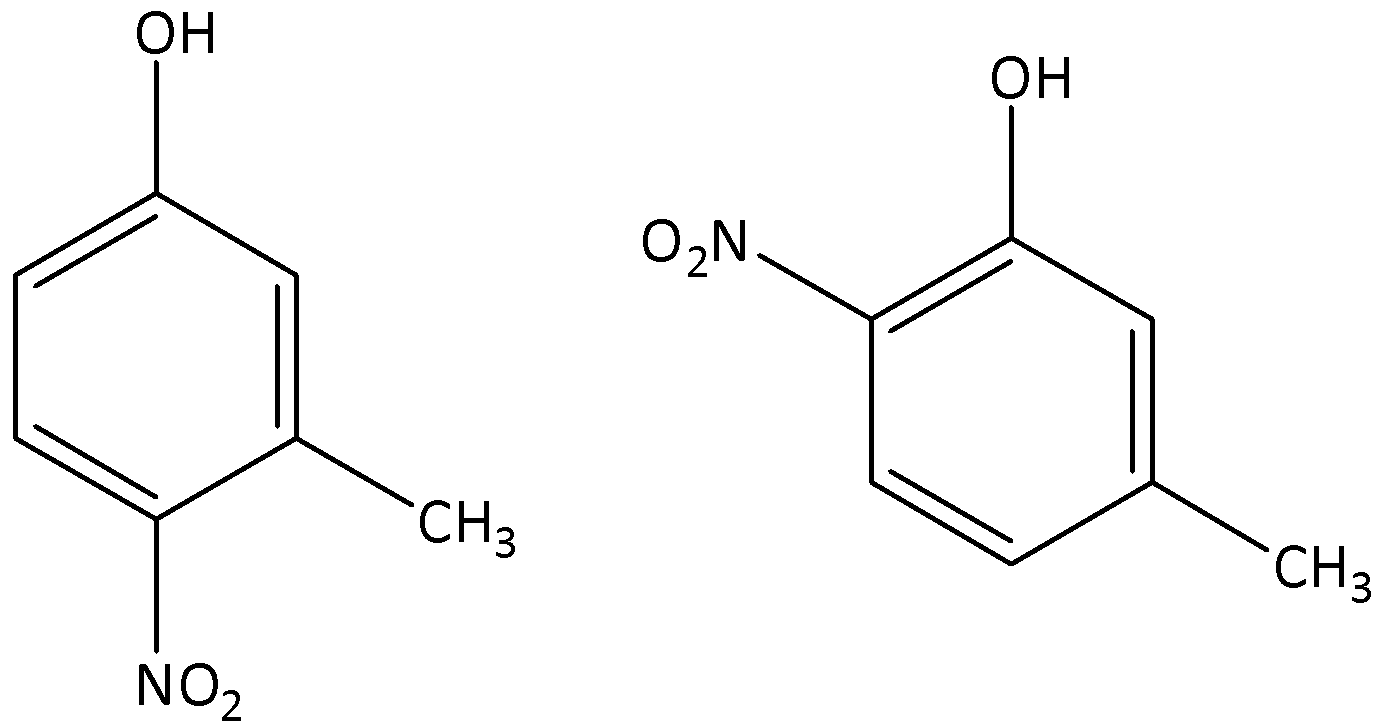
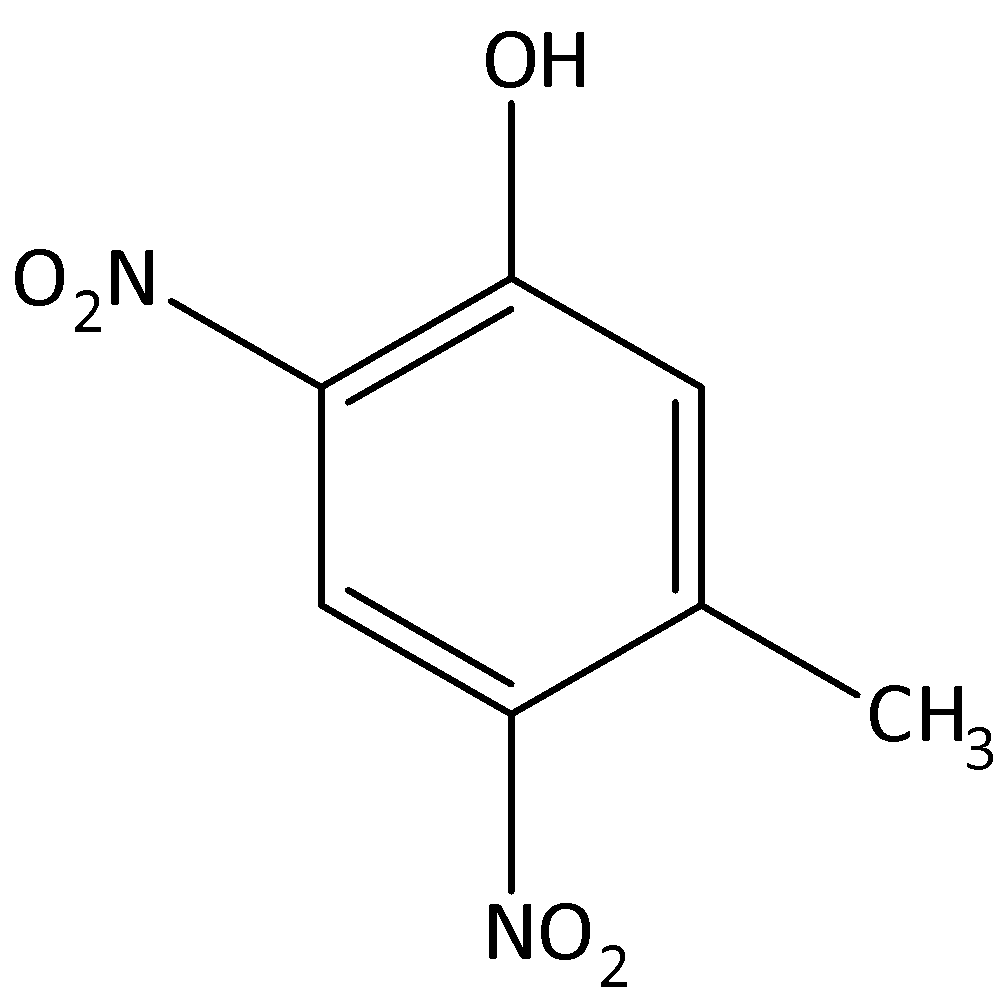
The major products formed by the dinitration of 3-methylphenol.
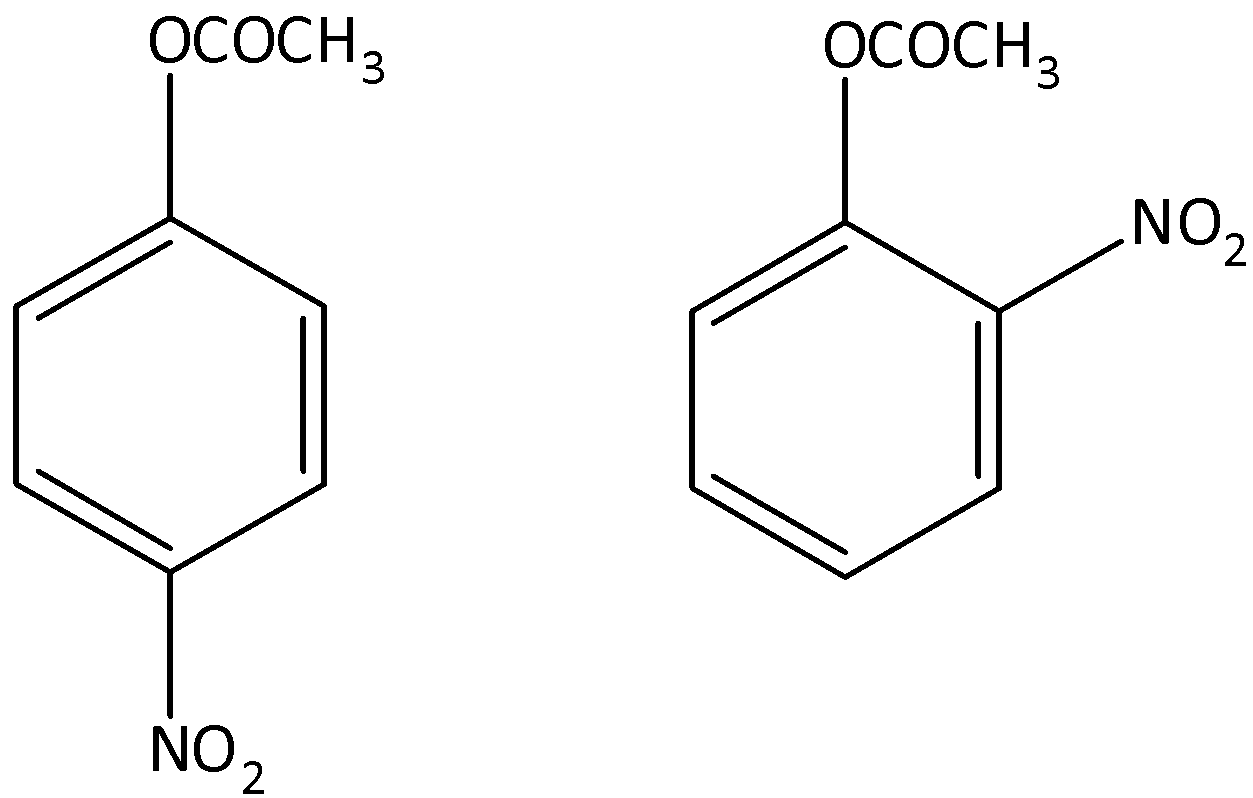
The major products formed by the mononitration of phenyl methanoate.
Note:
We have to know that phenyl methanoate is a compound that has less life and is unstable. Its nitration is not feasible to take place. But when the nitration is still done, product formed would be meta. In such cases, an important role is played by properties of the compounds.
Complete answer:
We know that in case of substituted aromatic compounds, the functional group present in the compound directs the next incoming group to a specific position in the aromatic ring. We call this as the directive influence of the group already bonded to the benzene ring.
If a monosubstituted compound is treated with an electrophile, it will undergo electrophilic aromatic substitution reaction and form a disubstituted compound which may be recognized using the descriptors ortho, meta, and para. If the relative yield of the ortho product and the para products are greater than that of the meta product, then the substituent found in the monosubstituted ring is named an ortho, para directing group.
We know that the methyl group and hydroxyl present in the benzene ring is ortho and para directing. Since the methyl group activates the ring at the para and ortho position much more than the meta positions, the electrophilic substitution reactions occur at these positions.
The influence of hydroxyl group and methyl group decides the incoming group position.
The major products formed by the mononitration of 3-methylphenol.

The major products formed by the dinitration of 3-methylphenol.

The major products formed by the mononitration of phenyl methanoate.

Note:
We have to know that phenyl methanoate is a compound that has less life and is unstable. Its nitration is not feasible to take place. But when the nitration is still done, product formed would be meta. In such cases, an important role is played by properties of the compounds.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




