
Write the structure of the following compound: \[3 - \] oxopentanal.
Answer
564.9k+ views
Hint:To write the structure of the following compound \[3 - \] oxopentanal we will use the basics of nomenclature of organic compounds. Nomenclature is the naming of organic compounds using some set of rules. Here we will write the structure using the name of an organic compound.
Complete answer:
First, we will determine the number of carbon atoms in the parent chain or principal chain. As we know that one of the rules of nomenclature is to find the parent chain with the maximum number of carbon atoms and substituents. So here we have \[3 - \] oxopentanal which implies that the parent chain has \[5\] carbon atoms. So we can draw a parent chain as given below.
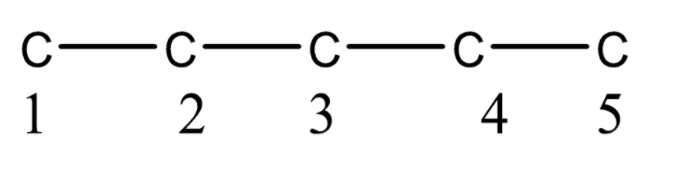
So now we have the numbered parent chain. Now we will find out if there is any functional group in the organic compound, if the functional group is present then it will have priority. As we have \[3 - \] oxopentanal which means aldehyde is present at the first carbon. So now the structure will be,
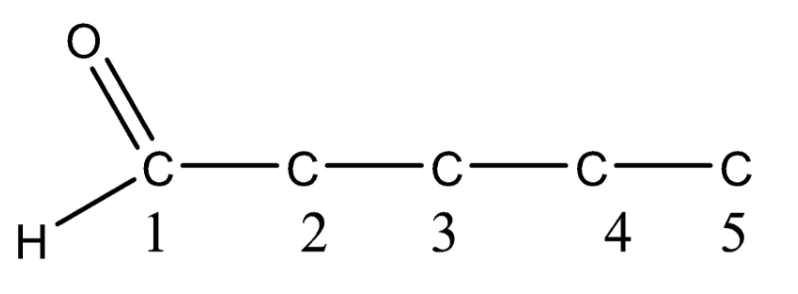
Now again consider the compound \[3 - \] oxopentanal. Here at \[C - 3\] the oxo group is present. So we can number the parent chain either from the right or left \[C - 3\] position will remain unchanged so the oxo group will be placed at \[C - 3\] in the parent chain or principal chain.
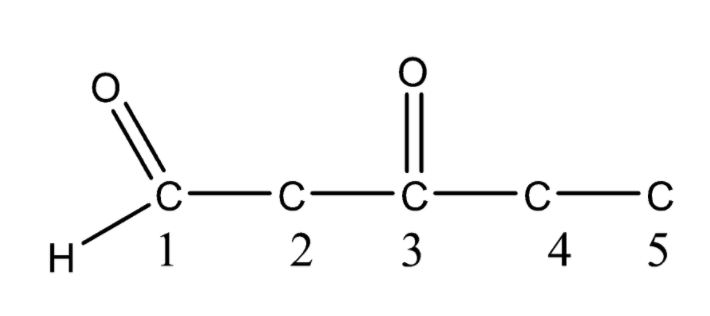
Now we have placed all the functional groups according to their position as given in the compound \[3 - \] oxopentanal. At last, we will complete the valency of each carbon atom and that structure will be the final structure of compound \[3 - \] oxopentanal.
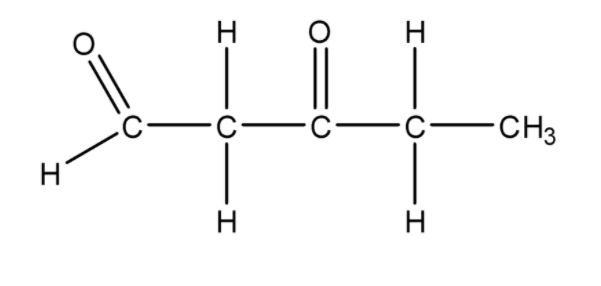
Therefore, the structure for \[3 - \] oxopentanal is
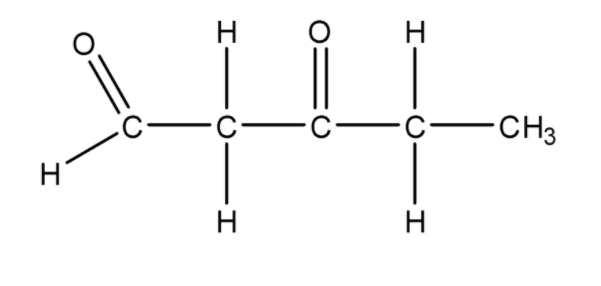 .
.
Note:
The molecular formula of \[3 - \] oxopentanal is \[{C_5}{H_8}{O_2}\]. The synonym for \[3 - \] oxopentanal is \[1,3\] pentanedione.
We can check our answer by using the reverse process we can name the chemical structure using the set of rules for the nomenclature of the organic compound.
Complete answer:
First, we will determine the number of carbon atoms in the parent chain or principal chain. As we know that one of the rules of nomenclature is to find the parent chain with the maximum number of carbon atoms and substituents. So here we have \[3 - \] oxopentanal which implies that the parent chain has \[5\] carbon atoms. So we can draw a parent chain as given below.

So now we have the numbered parent chain. Now we will find out if there is any functional group in the organic compound, if the functional group is present then it will have priority. As we have \[3 - \] oxopentanal which means aldehyde is present at the first carbon. So now the structure will be,
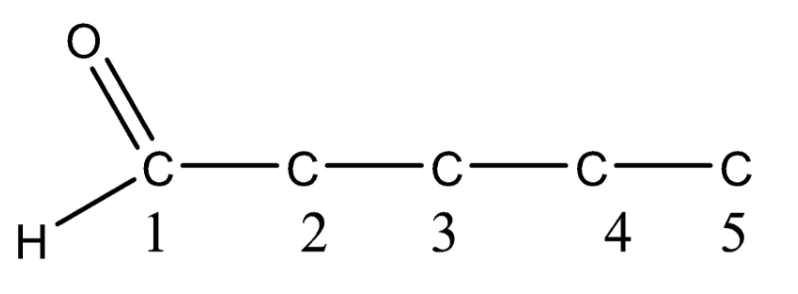
Now again consider the compound \[3 - \] oxopentanal. Here at \[C - 3\] the oxo group is present. So we can number the parent chain either from the right or left \[C - 3\] position will remain unchanged so the oxo group will be placed at \[C - 3\] in the parent chain or principal chain.

Now we have placed all the functional groups according to their position as given in the compound \[3 - \] oxopentanal. At last, we will complete the valency of each carbon atom and that structure will be the final structure of compound \[3 - \] oxopentanal.

Therefore, the structure for \[3 - \] oxopentanal is

Note:
The molecular formula of \[3 - \] oxopentanal is \[{C_5}{H_8}{O_2}\]. The synonym for \[3 - \] oxopentanal is \[1,3\] pentanedione.
We can check our answer by using the reverse process we can name the chemical structure using the set of rules for the nomenclature of the organic compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




