
Write the structure of n - methylethanamine
Answer
587.7k+ views
Hint: The derivatives of amine are formed by substituting the hydrogen atoms that are attached to the nitrogen. This makes the nitrogen either 1 – degree, 2 – degree or 3 – degree depending on the number of hydrogens that have been substituted.
Complete step by step answer:
-The parent compound in this given compound can be understood by the suffix added at the end of the name of the compound. As we can see, the name of the given compound ends with suffix -amine.
-Hence, the parent compound is Amine in this question. The structure of amine can be given as:
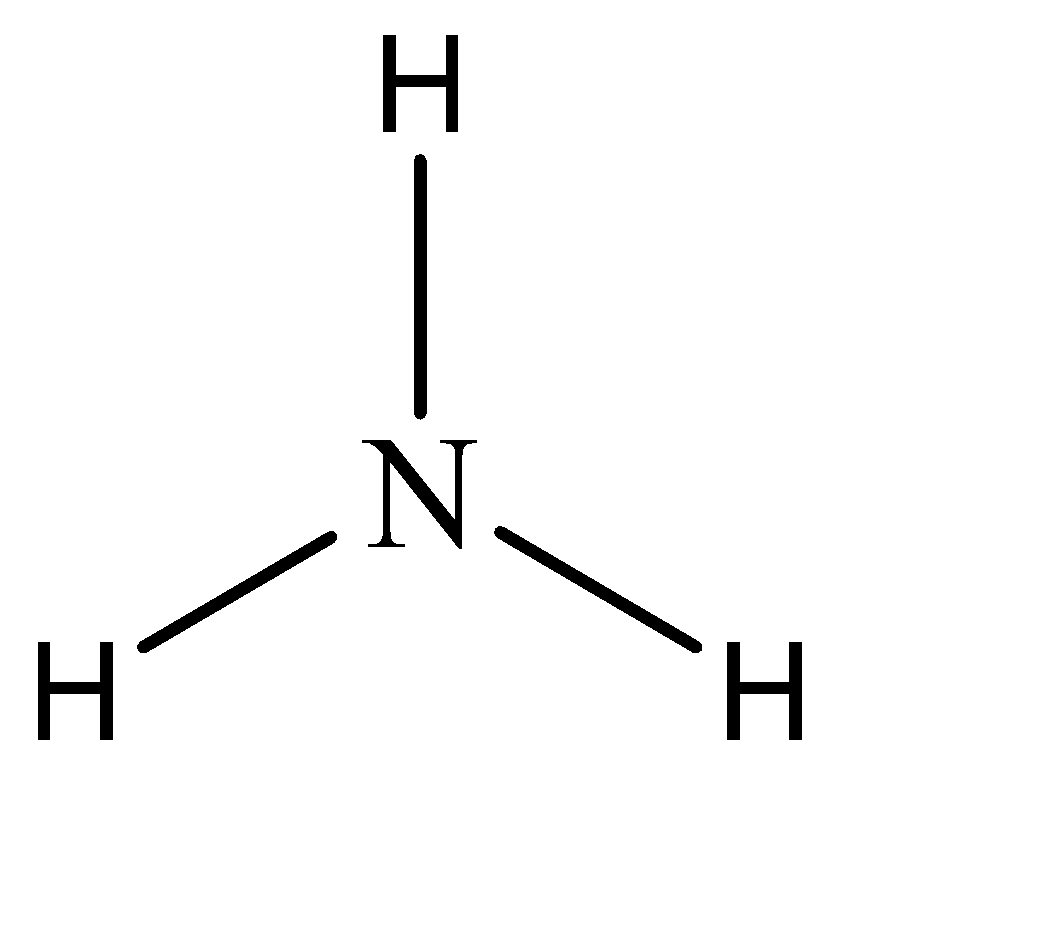
Now, while naming derivative compounds of amine, the rules that are followed include to place the corresponding replacing functional groups in alphabetical order followed by the suffix ‘amine’.
-The name of the given compound is n – methylethanamine. This means that two of the hydrogen atoms are substituted from amine. The functional groups that have substituted these hydrogen atoms are ethyl and methyl. Hence the structure of n – methylethanamine can be given as:
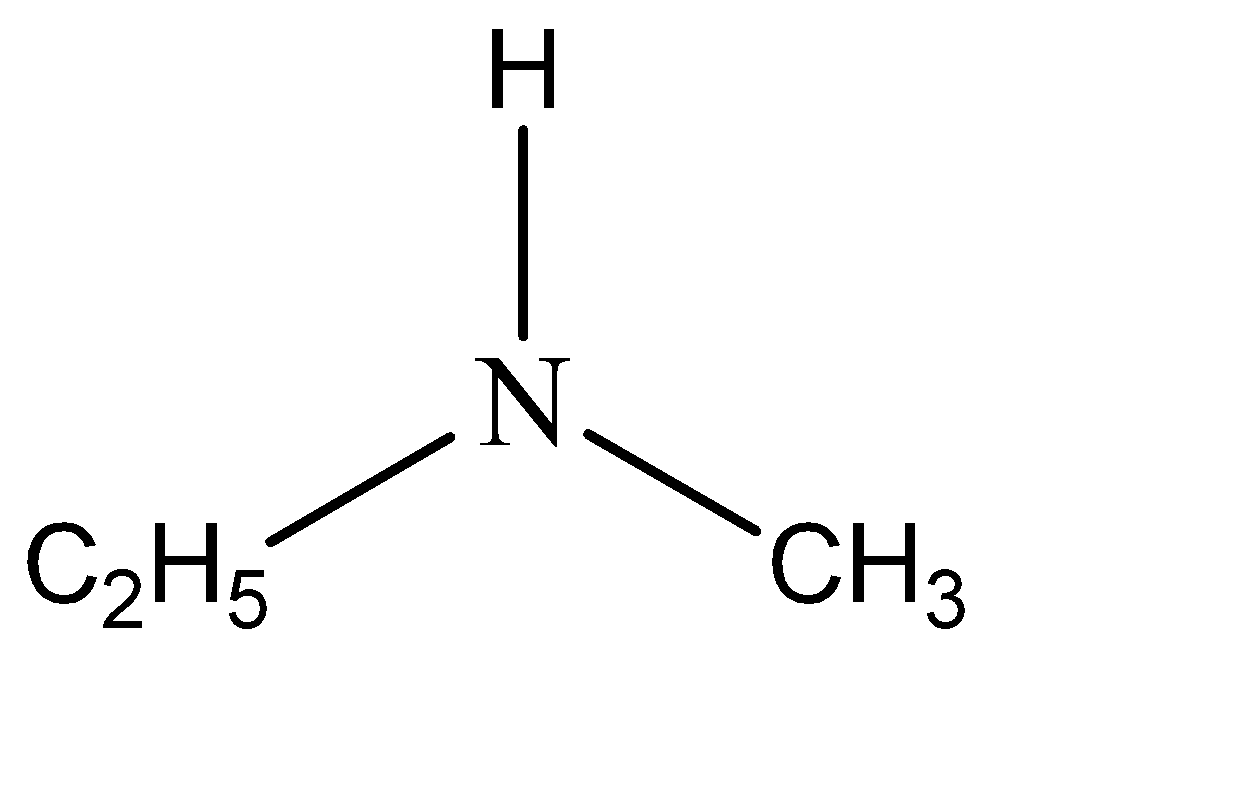
Note:
The terms primary (1º), secondary (2º) & tertiary (3º) are used to classify amines in a completely different manner than they were used for alcohols or alkyl halides. When applied to amines these terms refer to the number of alkyl (or aryl) substituents bonded to the nitrogen atom, whereas in other cases they refer to the nature of an alkyl group.
Complete step by step answer:
-The parent compound in this given compound can be understood by the suffix added at the end of the name of the compound. As we can see, the name of the given compound ends with suffix -amine.
-Hence, the parent compound is Amine in this question. The structure of amine can be given as:

Now, while naming derivative compounds of amine, the rules that are followed include to place the corresponding replacing functional groups in alphabetical order followed by the suffix ‘amine’.
-The name of the given compound is n – methylethanamine. This means that two of the hydrogen atoms are substituted from amine. The functional groups that have substituted these hydrogen atoms are ethyl and methyl. Hence the structure of n – methylethanamine can be given as:

Note:
The terms primary (1º), secondary (2º) & tertiary (3º) are used to classify amines in a completely different manner than they were used for alcohols or alkyl halides. When applied to amines these terms refer to the number of alkyl (or aryl) substituents bonded to the nitrogen atom, whereas in other cases they refer to the nature of an alkyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




