
Write the name and structures of monomers of the following polymers.
(i) Terylene
(ii) Buna – S
(iii) Neoprene
Answer
585.3k+ views
Hint: Polymers are the high molecular mass compounds whose structure are composed of a large number of simple repeating units. The repeating structural units are usually obtained from low molecular mass of simple compounds called monomers.
Complete step by step answer:
A monomer is a molecule that forms the basic unit of polymers, which are the building block of proteins. Monomers bind to other monomers to form repeating chain molecules through a process known as polymerisation. Monomers may be natural or synthetic.
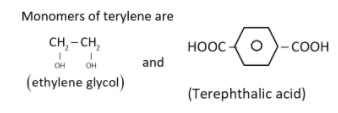
(i) Terylene: It is obtained by condensation polymerisation of ethylene glycol (1,2 - ethanediol) and terephthalic acid:
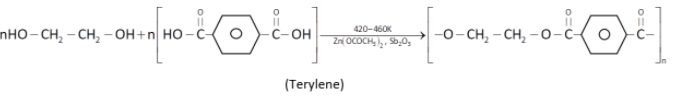
Uses: (i) Terylene fiber is used as polyester tricot knit as a fashion garments fabric.
(ii) Used for the laundry usage as an automatic clothing vacuum packaging machine.
(iii) It is used to make nonwoven needle punched carpet particularly for the exhibition use.
(2) Buna–S: It is a co–polymer of butadiene and styrene. It is prepared by copolymerisation of 1,3 - butadiene and styrene along with sodium
\[\mathop {nC{H_2} = CH - CH = C{H_2}}\limits_{\left( {1,3 - butadiene} \right)} + nC{H_2} = CH - \mathop {{C_6}{H_5}}\limits_{styrene} \xrightarrow{{Na,{\text{ heat}}}}{\left[ { - C{H_2} - CH = CH - C{H_2} - C{H_2} - \mathop {\mathop {CH}\limits_| }\limits_{{C_6}{H_5}} - } \right]_n}\]
Monomers of Buna–S are $\mathop {C{H_2} = CH - CH = C{H_2}}\limits_{\left( {1,3 - butadiene} \right)} $ and $\mathop {C{H_2} = CH - {C_6}{H_5}}\limits_{\left( {Styrene} \right)} $
Uses: (i) It is used widely in pneumatic tires in shoe heels and soles, gaskets and even chewing gum.
(ii) It is extensively used in coated papers, being one of the most cost effective resins to bind pigmented coatings.
(iii) It is a commodity material which competes with natural rubber.
(3) Neoprene: Neoprene is the group of rubbers which are formed by the process of polymerisation of chloroprene
$\mathop {nC{H_2} = \mathop {\mathop C\limits_| }\limits_{Cl} - CH = C{H_2}\xrightarrow{{h\nu }}}\limits_{\left( {Chloroprene} \right)} \mathop {{{\left[ { - C{H_2} - \mathop {\mathop C\limits_| }\limits_{Cl} = CH - C{H_2} - } \right]}_n}}\limits_{\left( {neoprene} \right)} $
Monomer of neoprene is chloroprene$\left( {C{H_2} = \mathop {\mathop C\limits_| }\limits_{Cl} - CH = C{H_2}} \right)$
Uses: (i) Sports and medical equipment.
(ii) Laptop covers and sleeves.
(iii) Wetsuits, dry suits and waders.
Note: Polymers are important compounds of our daily life and they may be natural, semi synthetic or synthetic. But these compounds are economically good and are a better substitute. In this question the mentioned polymer is used in various fields with high durability.
Complete step by step answer:
A monomer is a molecule that forms the basic unit of polymers, which are the building block of proteins. Monomers bind to other monomers to form repeating chain molecules through a process known as polymerisation. Monomers may be natural or synthetic.
(i) Terylene: It is obtained by condensation polymerisation of ethylene glycol (1,2 - ethanediol) and terephthalic acid:


Uses: (i) Terylene fiber is used as polyester tricot knit as a fashion garments fabric.
(ii) Used for the laundry usage as an automatic clothing vacuum packaging machine.
(iii) It is used to make nonwoven needle punched carpet particularly for the exhibition use.
(2) Buna–S: It is a co–polymer of butadiene and styrene. It is prepared by copolymerisation of 1,3 - butadiene and styrene along with sodium
\[\mathop {nC{H_2} = CH - CH = C{H_2}}\limits_{\left( {1,3 - butadiene} \right)} + nC{H_2} = CH - \mathop {{C_6}{H_5}}\limits_{styrene} \xrightarrow{{Na,{\text{ heat}}}}{\left[ { - C{H_2} - CH = CH - C{H_2} - C{H_2} - \mathop {\mathop {CH}\limits_| }\limits_{{C_6}{H_5}} - } \right]_n}\]
Monomers of Buna–S are $\mathop {C{H_2} = CH - CH = C{H_2}}\limits_{\left( {1,3 - butadiene} \right)} $ and $\mathop {C{H_2} = CH - {C_6}{H_5}}\limits_{\left( {Styrene} \right)} $
Uses: (i) It is used widely in pneumatic tires in shoe heels and soles, gaskets and even chewing gum.
(ii) It is extensively used in coated papers, being one of the most cost effective resins to bind pigmented coatings.
(iii) It is a commodity material which competes with natural rubber.
(3) Neoprene: Neoprene is the group of rubbers which are formed by the process of polymerisation of chloroprene
$\mathop {nC{H_2} = \mathop {\mathop C\limits_| }\limits_{Cl} - CH = C{H_2}\xrightarrow{{h\nu }}}\limits_{\left( {Chloroprene} \right)} \mathop {{{\left[ { - C{H_2} - \mathop {\mathop C\limits_| }\limits_{Cl} = CH - C{H_2} - } \right]}_n}}\limits_{\left( {neoprene} \right)} $
Monomer of neoprene is chloroprene$\left( {C{H_2} = \mathop {\mathop C\limits_| }\limits_{Cl} - CH = C{H_2}} \right)$
Uses: (i) Sports and medical equipment.
(ii) Laptop covers and sleeves.
(iii) Wetsuits, dry suits and waders.
Note: Polymers are important compounds of our daily life and they may be natural, semi synthetic or synthetic. But these compounds are economically good and are a better substitute. In this question the mentioned polymer is used in various fields with high durability.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




