
Write the molecular formulae for the following compounds and name the elements present.
Sulphuric acid
Answer
519k+ views
Hint: The chemical symbols for the constituent elements are accompanied by numeric subscripts defining the number of atoms of each element found in the molecule in a molecular formula. The empirical formula describes the compound's simplest whole-integer atom ratio.
Complete answer:
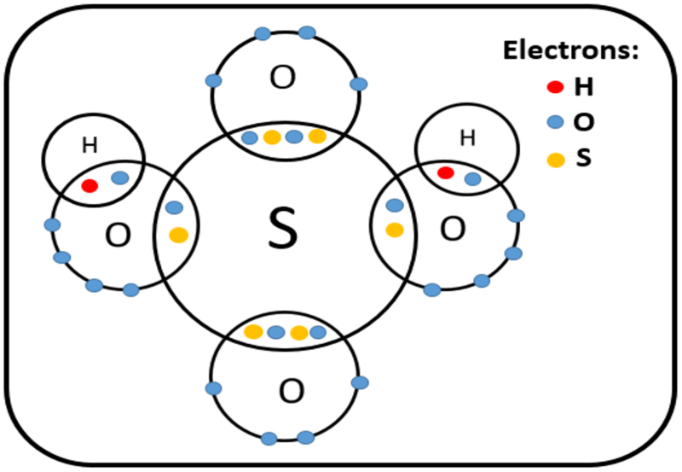
Sulphuric acid, also known as oil of vitriol, is a mineral acid with the molecular formula $H_2SO_4$ that is made up of the elements sulphur, oxygen, and hydrogen. It is a colourless, odourless, viscous liquid that is miscible in all proportions of water. Since it is an oxidant which has a deep acidic disposition, the acid in its pure form is extremely corrosive to other products. Furthermore, pure acid is a dehydrator, meaning it can remove water from whatever material it comes into contact with (except phosphorus pentoxide that dehydrates sulfuric acid to sulphur trioxide). It's also hygroscopic, so it can capture water vapour from the air quickly.
A lot of heat is released as sulfuric acid is added to water (this can not be reversed). Pure sulfuric acid can cause serious chemical burns and even secondary thermal burns due to dehydration as it comes into contact with it; even minor concentrations of the pure acid can be fatal. Although the solution of sulfuric acid in water is much less toxic, the oxidative and dehydrating effects are only present in the pure acid, the solution of the acid in water would also be very acidic and should be treated with caution.
Elements present in Sulphuric Acid $H_2SO_4$ = Hydrogen, sulphur and oxygen.
Note:
In the chemical industry, sulfuric acid is also essential. It's most common in fertiliser production, but it's also used in mineral processing, oil refining, wastewater treatment, and chemical synthesis. It's used in a variety of products, from acidic drain cleaners for the home, as an electrolyte in lead-acid batteries, to dehydrate a compound, and as a washing agent.
Complete answer:
Sulphuric acid, also known as oil of vitriol, is a mineral acid with the molecular formula $H_2SO_4$ that is made up of the elements sulphur, oxygen, and hydrogen. It is a colourless, odourless, viscous liquid that is miscible in all proportions of water. Since it is an oxidant which has a deep acidic disposition, the acid in its pure form is extremely corrosive to other products. Furthermore, pure acid is a dehydrator, meaning it can remove water from whatever material it comes into contact with (except phosphorus pentoxide that dehydrates sulfuric acid to sulphur trioxide). It's also hygroscopic, so it can capture water vapour from the air quickly.
A lot of heat is released as sulfuric acid is added to water (this can not be reversed). Pure sulfuric acid can cause serious chemical burns and even secondary thermal burns due to dehydration as it comes into contact with it; even minor concentrations of the pure acid can be fatal. Although the solution of sulfuric acid in water is much less toxic, the oxidative and dehydrating effects are only present in the pure acid, the solution of the acid in water would also be very acidic and should be treated with caution.
Elements present in Sulphuric Acid $H_2SO_4$ = Hydrogen, sulphur and oxygen.

Note:
In the chemical industry, sulfuric acid is also essential. It's most common in fertiliser production, but it's also used in mineral processing, oil refining, wastewater treatment, and chemical synthesis. It's used in a variety of products, from acidic drain cleaners for the home, as an electrolyte in lead-acid batteries, to dehydrate a compound, and as a washing agent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




