
Write the mechanism of the following reaction:
\[2C{H_3}C{H_2}OH\mathop \to \limits^{{H^ + }} C{H_3}C{H_2}OC{H_2}C{H_3}\]
Answer
585.3k+ views
Hint: Heating alcohols in presence of concentrated sulphuric acid that results in condensation of two alcohol molecules along with elimination of water molecules and formation of ether. In such a reaction, sulphuric acid acts as a catalyst and it is a protic acid, releasing hydrogen ions in aqueous medium.
Complete step by step answer:
Diethyl ether belongs to an ether functional group of organic compounds. It is colourless, volatile and has a sweet solvent-like smell. It is a very common solvent used in laboratories. It can be prepared in laboratories and industries as well, known as acid ether synthesis.
$C{H_3}C{H_2}OH\xrightarrow{{concd{H_2}s{o_4}}}C{H_2}C{H_3} + {H_2}O$
Step 1: Ethanol is mixed with a strong acid such as sulphuric acid such that the acid dissociates in the aqueous environment and produces hydronium ions or protons. Soon after that, we will see that the proton or hydrogen ion protonated the highly electronegative oxygen atom of ethanol, giving positive charge to the ethanol.
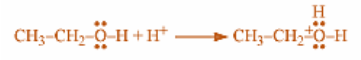
Step 2: Now, a nucleophilic oxygen atom of deprotonated ethanol eliminates a water molecule from another protonated ethanol molecule which is electrophilic in nature, produces diethyl ether with positively charged hydrogen on its oxygen.
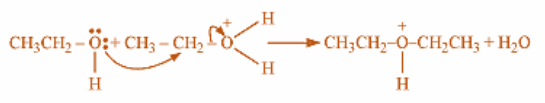
Last step involves removal of proton and marking sulphuric acid as a catalyst used in this reaction as it is retained back in the process. This reaction must take place at temperatures lower than 150-degree Celsius to guarantee that the product ethylene is not the final product formed in the reaction. Because we know that only high temperatures can eliminate water and dehydrate the ethanol to ethylene.
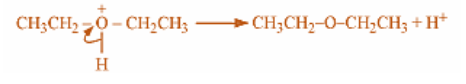
This is the mechanism followed for synthesis of diethyl ether.
Note:
Synthesis of diethyl ether from ethanol is a reversible reaction as there exists an equilibrium between reactants and products. Therefore, for good yield of ether, it should be distilled out of the reaction mixture before it gets reverted to ethanol.
Complete step by step answer:
Diethyl ether belongs to an ether functional group of organic compounds. It is colourless, volatile and has a sweet solvent-like smell. It is a very common solvent used in laboratories. It can be prepared in laboratories and industries as well, known as acid ether synthesis.
$C{H_3}C{H_2}OH\xrightarrow{{concd{H_2}s{o_4}}}C{H_2}C{H_3} + {H_2}O$
Step 1: Ethanol is mixed with a strong acid such as sulphuric acid such that the acid dissociates in the aqueous environment and produces hydronium ions or protons. Soon after that, we will see that the proton or hydrogen ion protonated the highly electronegative oxygen atom of ethanol, giving positive charge to the ethanol.
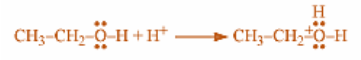
Step 2: Now, a nucleophilic oxygen atom of deprotonated ethanol eliminates a water molecule from another protonated ethanol molecule which is electrophilic in nature, produces diethyl ether with positively charged hydrogen on its oxygen.

Last step involves removal of proton and marking sulphuric acid as a catalyst used in this reaction as it is retained back in the process. This reaction must take place at temperatures lower than 150-degree Celsius to guarantee that the product ethylene is not the final product formed in the reaction. Because we know that only high temperatures can eliminate water and dehydrate the ethanol to ethylene.

This is the mechanism followed for synthesis of diethyl ether.
Note:
Synthesis of diethyl ether from ethanol is a reversible reaction as there exists an equilibrium between reactants and products. Therefore, for good yield of ether, it should be distilled out of the reaction mixture before it gets reverted to ethanol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




