
Write the IUPAC name and structural formula of the following: ${C_3}{H_4}$
Answer
501.6k+ views
Hint: We know that the chemical name of any organic or inorganic compound is given according to the rules set by IUPAC (International Union of Pure and Applied Chemistry). And to determine the name and structure of the compound using molecular formula, we should know about its double bond equivalent and any other functional groups that are present in the given compound.
Complete answer:
IUPAC (International Union of Pure and Applied Chemistry) has set some rules that need to be strictly followed while naming an organic, inorganic or any chemical compound.
First of all, to determine the structure and IUPAC name of the compound, we need to find the double bond equivalent for that particular compound.
The formula for calculating D.B.E. for any compound is given as:
$D.B.E = C + 1 - \dfrac{H}{2} - \dfrac{X}{2} + \dfrac{N}{2}$
Where, C represents the number of carbon atoms, H represents the number of hydrogen atoms, X represents the number of halogen atoms and N represents the number of nitrogen atoms in the given compound.
In the given compound, ${C_3}{H_4}$ there are three carbon atoms and four hydrogen atoms.
$$D.B.E = 3 + 1 - \dfrac{4}{2} - \dfrac{0}{2} + \dfrac{0}{2}$$
$D.B.E = 2$
So, in this compound there will be one double bond and one ring in the structure of the compound.
The structure of ${C_3}{H_4}$ is found to be:
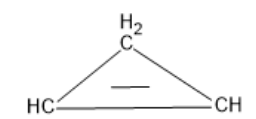
The name of the compound is cyclopropene.
Note:
For the functional groups, names are written in the short form. For example, if there is an alcoholic group in the compound, then suffix “-ol” is added to its name. For aldehyde, the suffix “-al” is added and for the ketonic group, the suffix added to the name of the compound is “-one”. For carboxylic acid, the suffix is “-oic acid”.
Complete answer:
IUPAC (International Union of Pure and Applied Chemistry) has set some rules that need to be strictly followed while naming an organic, inorganic or any chemical compound.
First of all, to determine the structure and IUPAC name of the compound, we need to find the double bond equivalent for that particular compound.
The formula for calculating D.B.E. for any compound is given as:
$D.B.E = C + 1 - \dfrac{H}{2} - \dfrac{X}{2} + \dfrac{N}{2}$
Where, C represents the number of carbon atoms, H represents the number of hydrogen atoms, X represents the number of halogen atoms and N represents the number of nitrogen atoms in the given compound.
In the given compound, ${C_3}{H_4}$ there are three carbon atoms and four hydrogen atoms.
$$D.B.E = 3 + 1 - \dfrac{4}{2} - \dfrac{0}{2} + \dfrac{0}{2}$$
$D.B.E = 2$
So, in this compound there will be one double bond and one ring in the structure of the compound.
The structure of ${C_3}{H_4}$ is found to be:

The name of the compound is cyclopropene.
Note:
For the functional groups, names are written in the short form. For example, if there is an alcoholic group in the compound, then suffix “-ol” is added to its name. For aldehyde, the suffix “-al” is added and for the ketonic group, the suffix added to the name of the compound is “-one”. For carboxylic acid, the suffix is “-oic acid”.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




