
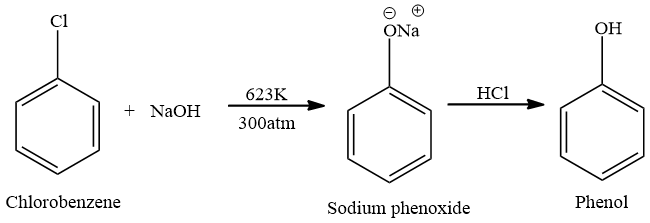
Write the chemical reaction for the preparation of phenol from chlorobenzene.
Answer
592.2k+ views
Hint: Chlorobenzene has partial double bond character. Removal of chlorine atom from benzene rings requires high temperature and high pressure.
Complete answer:
Phenol is an organic compound containing a benzene ring bonded to the hydroxyl group.Phenol are weak acids and generally form phenoxide ions by losing one positive hydrogen ion from the hydroxyl group.
Chlorobenzene is formed by monochlorination of the benzene ring. To form chloro benzene, the Benzene ring is reacted with chlorine gas and $FeC{{l}_{3}}$. The lone pair of electrons present on chlorine atoms are in conjugation with the pie electrons of the benzene ring, therefore, Chlorobenzene is having a double bond character therefore, removal of chlorine atom from benzene is not easy. Removal of chlorine from benzene atoms requires high pressure and temperature,because chlorobenzene is less reactive and does not undergo nucleophilic substitution reaction easily. Therefore, for removal of chlorine atom, Chlorobenzene is reacted with Sodium Hydroxide at high pressure of 300 atm and high temperature of 623K. This results in the formation of sodium phenoxide. Sodium phenoxide on acidification with HCl results in the formation of Phenol.
Note: When the electron withdrawing group is attached to the benzene ring, the removal of chlorine group becomes easy. When an electron withdrawing group like Nitro group, cyano group is attached to the ortho or para position of chlorobenzene. Trinitro chlorobenzene is highly reactive and gets hydrolysed at room temperature.
Complete answer:
Phenol is an organic compound containing a benzene ring bonded to the hydroxyl group.Phenol are weak acids and generally form phenoxide ions by losing one positive hydrogen ion from the hydroxyl group.
Chlorobenzene is formed by monochlorination of the benzene ring. To form chloro benzene, the Benzene ring is reacted with chlorine gas and $FeC{{l}_{3}}$. The lone pair of electrons present on chlorine atoms are in conjugation with the pie electrons of the benzene ring, therefore, Chlorobenzene is having a double bond character therefore, removal of chlorine atom from benzene is not easy. Removal of chlorine from benzene atoms requires high pressure and temperature,because chlorobenzene is less reactive and does not undergo nucleophilic substitution reaction easily. Therefore, for removal of chlorine atom, Chlorobenzene is reacted with Sodium Hydroxide at high pressure of 300 atm and high temperature of 623K. This results in the formation of sodium phenoxide. Sodium phenoxide on acidification with HCl results in the formation of Phenol.

Note: When the electron withdrawing group is attached to the benzene ring, the removal of chlorine group becomes easy. When an electron withdrawing group like Nitro group, cyano group is attached to the ortho or para position of chlorobenzene. Trinitro chlorobenzene is highly reactive and gets hydrolysed at room temperature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




