
Write the chemical equation for the oxidation of cyclohexene with acidified \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] and also give IUPAC name of the compound formed.
Answer
589.8k+ views
Hint: IUPAC nomenclature is used to make an international standard of naming molecules to help in communication . According to the IUPAC nomenclature, every chemical compound can be named unique and can be given a unique structure. A certain set of rules must be followed to name the compound.
Complete step by step answer:
A chemical equation occurs when the number of atoms involved in the reactants side is equal to the number of atoms in the product side.
Reagent can be a substance or mixture of compounds which are basically used in chemical analysis or reactions. When a reagent is added to a system it causes chemical reactions. For a particular reaction a particular reagent gets consumed in the process of the chemical reaction.
Now when acidified \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] reacts with cyclohexene oxidation of the double bonds takes place as follows,
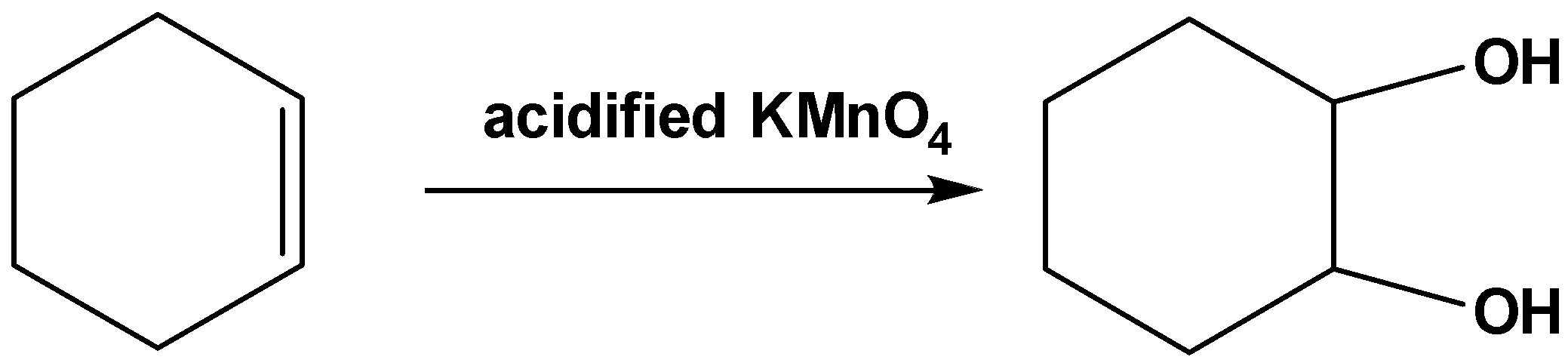
Now the IUPAC name of this product is cyclohexane-1,2-diol.
Additional information:
-While naming cyclic organic compounds, certain nomenclature rules need to be followed:
-At first determine the cyclic organic compound or the “cyclo” to be used as the primary prefix.
-In a situation where the compound contains an alkyl group, in case the alkyl group has a higher number of carbon atoms, then it would be treated as the parent chain. Otherwise, the cycloalkane would be the parent chain.
-Next, name the functional groups in their alphabetical order.
-The naming should be done in such a way that the carbon on which the functional group is attached should have the lowest number in nomenclature.
-If the same functional group is repeated more than once, then corresponding prefixes along with the correct position numbering of the functional group must be mentioned.
-After all these functional groups are named, then the name of the cycloalkane can be written in the end. The name of the cycloalkane depends on the number of carbon atoms present in the cyclic compound.
Note:
In IUPAC nomenclature, the root word is basically the number of total carbons in a longest chain of that compound.
… and so on.
The primary suffix is basically used to differentiate between the saturated compounds( Alkanes) and unsaturated compounds (Alkene and Alkynes).
If there are more than one suffix. Then one of those suffixes is considered a secondary suffix. Example: Methanol (Alkanol) , here ‘ol’ is a secondary suffix.
Complete step by step answer:
A chemical equation occurs when the number of atoms involved in the reactants side is equal to the number of atoms in the product side.
Reagent can be a substance or mixture of compounds which are basically used in chemical analysis or reactions. When a reagent is added to a system it causes chemical reactions. For a particular reaction a particular reagent gets consumed in the process of the chemical reaction.
Now when acidified \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] reacts with cyclohexene oxidation of the double bonds takes place as follows,

Now the IUPAC name of this product is cyclohexane-1,2-diol.
Additional information:
-While naming cyclic organic compounds, certain nomenclature rules need to be followed:
-At first determine the cyclic organic compound or the “cyclo” to be used as the primary prefix.
-In a situation where the compound contains an alkyl group, in case the alkyl group has a higher number of carbon atoms, then it would be treated as the parent chain. Otherwise, the cycloalkane would be the parent chain.
-Next, name the functional groups in their alphabetical order.
-The naming should be done in such a way that the carbon on which the functional group is attached should have the lowest number in nomenclature.
-If the same functional group is repeated more than once, then corresponding prefixes along with the correct position numbering of the functional group must be mentioned.
-After all these functional groups are named, then the name of the cycloalkane can be written in the end. The name of the cycloalkane depends on the number of carbon atoms present in the cyclic compound.
Note:
In IUPAC nomenclature, the root word is basically the number of total carbons in a longest chain of that compound.
| No. of carbons | Root word |
| 1 | meth |
| 2 | eth |
| 3 | prop |
| 4 | but |
| 5 | pent |
… and so on.
The primary suffix is basically used to differentiate between the saturated compounds( Alkanes) and unsaturated compounds (Alkene and Alkynes).
| compound | suffix |
| Alkane | Ane |
| Alkene | Ene |
| Alkyne | Yne |
If there are more than one suffix. Then one of those suffixes is considered a secondary suffix. Example: Methanol (Alkanol) , here ‘ol’ is a secondary suffix.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




