
Write short notes on:
(I) Activation energy
(ii) Arrhenius equation
Answer
585.3k+ views
Hint: The concept of the above equation and energy is evolved from chemical kinetics.
Complete step by step solution: rate constant in chemical kinetics is affected by temperature, nature of reactant and catalyst. Threshold energy is the minimum amount of energy at which reaction takes place.
Activation energy: the energy given to the reactant to reach the threshold value of energy is called activation energy.
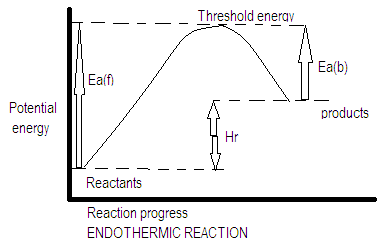
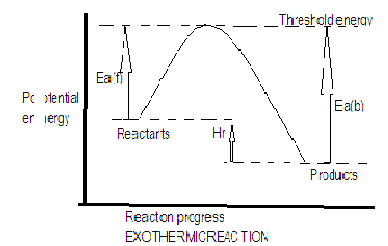
Activation energy is different for both exothermic and endothermic reactions. For endothermic reaction as shown in the diagram Ea(f) is the activation energy for forward reaction and Ea(b) is the activation energy for backward reaction. Enthalpy of a reaction$\Delta {{\rm H}_r} = {E_{{a_f}}} - {E_{{a_b}}}$ . In an endothermic reaction reactants have a lower potential energy as compared to the products because we know that heat is released during endothermic reaction whereas in exothermic reaction heat is absorbed hence the potential energy of the reactants is greater than the potential energy of the products.
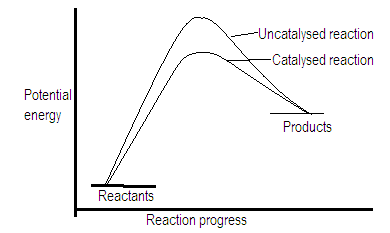
Positive catalyst lowers the activation energy of the reaction by changing the reaction path and hence increases the rate of reaction.
(ii) Arrhenius equation: the rate constant of a reaction is a function of temperature. It is related to temperature by the Arrhenius equation.
$K = A{e^{\dfrac{{ - {E_a}}}{{RT}}}}$
Where k is the rate constant, A is the frequency factor or pre exponential factor or Arrhenius constant, T is the absolute temperature and Ea is the activation energy of the reaction in J/mole.
Taking log on both the sides in the above written Arrhenius equation we get:
Log K=log A-$\dfrac{{{E_a}}}{{2.303RT}} = \log \dfrac{{{k_1}}}{{{k_2}}} = \dfrac{{{E_a}}}{{2.303RT}}[\dfrac{1}{{{T_1}}} - \dfrac{1}{{{T_2}}}]$
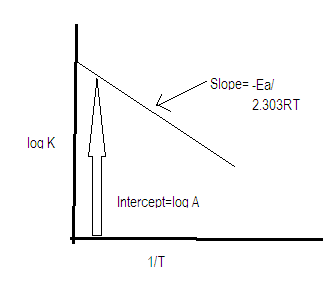
Note: In Arrhenius equation the factor ${e^{\dfrac{{ - {E_a}}}{{RT}}}}$is the fraction of effective collisions and A represents the number of total bimolecular collision taking place in unit volume and in unit time.
Complete step by step solution: rate constant in chemical kinetics is affected by temperature, nature of reactant and catalyst. Threshold energy is the minimum amount of energy at which reaction takes place.
Activation energy: the energy given to the reactant to reach the threshold value of energy is called activation energy.


Activation energy is different for both exothermic and endothermic reactions. For endothermic reaction as shown in the diagram Ea(f) is the activation energy for forward reaction and Ea(b) is the activation energy for backward reaction. Enthalpy of a reaction$\Delta {{\rm H}_r} = {E_{{a_f}}} - {E_{{a_b}}}$ . In an endothermic reaction reactants have a lower potential energy as compared to the products because we know that heat is released during endothermic reaction whereas in exothermic reaction heat is absorbed hence the potential energy of the reactants is greater than the potential energy of the products.

Positive catalyst lowers the activation energy of the reaction by changing the reaction path and hence increases the rate of reaction.
(ii) Arrhenius equation: the rate constant of a reaction is a function of temperature. It is related to temperature by the Arrhenius equation.
$K = A{e^{\dfrac{{ - {E_a}}}{{RT}}}}$
Where k is the rate constant, A is the frequency factor or pre exponential factor or Arrhenius constant, T is the absolute temperature and Ea is the activation energy of the reaction in J/mole.
Taking log on both the sides in the above written Arrhenius equation we get:
Log K=log A-$\dfrac{{{E_a}}}{{2.303RT}} = \log \dfrac{{{k_1}}}{{{k_2}}} = \dfrac{{{E_a}}}{{2.303RT}}[\dfrac{1}{{{T_1}}} - \dfrac{1}{{{T_2}}}]$

Note: In Arrhenius equation the factor ${e^{\dfrac{{ - {E_a}}}{{RT}}}}$is the fraction of effective collisions and A represents the number of total bimolecular collision taking place in unit volume and in unit time.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




