
Write equations of the following reactions. a) Alkylation of anisole b) Nitration of anisole c) Acetylation of anisole.
Answer
518.1k+ views
Hint: Anisole is an organic compound having a benzene ring and a methoxy group at Para position. Anisole is aromatic in nature. Aromatic compounds undergo alkylation and acetylation through a special reaction called Friedal craft’s reaction. Nitration is the introduction of the nitro group in the ring of anisole.
Complete answer:
Anisole is an aromatic compound that has a benzene ring and a $\left( -OC{{H}_{3}} \right)$group at the para position. It is a type of aromatic ether that undergoes electrophilic substitution reaction. The substituted group gets attached at the ortho and para positions as aromatic ethers are ortho – para directing due to the lone pair in conjugation with the ring so ortho – para positions acquire partial negative charges.
a)Alkylation of anisole consist of Friedal craft alkylation, where alkyl halide and anhydrous aluminum chloride is used, the equation for the reaction is:
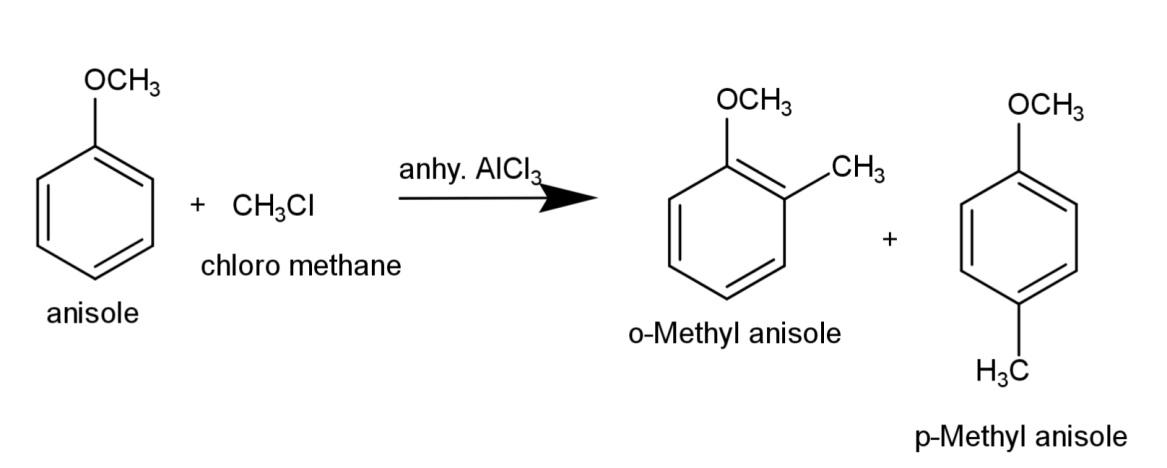
b) Nitration of anisole involves the reaction of anisole with the nitrating mixture that consist of concentrated sulfuric acid and nitric acid, that results in substitution of nitro group and the reaction is:
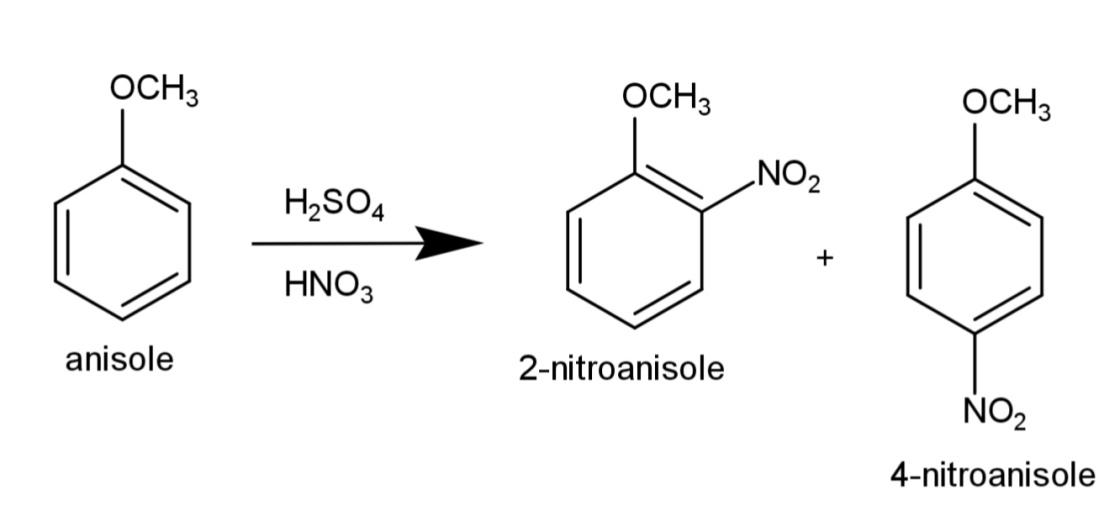
c) Acetylation of anisole consists of substitution of the acetyl group on anisole, the reaction involves Friedal craft acetylation that involves ethanoyl chloride in presence of anhydrous aluminum chloride, the reaction equation is:
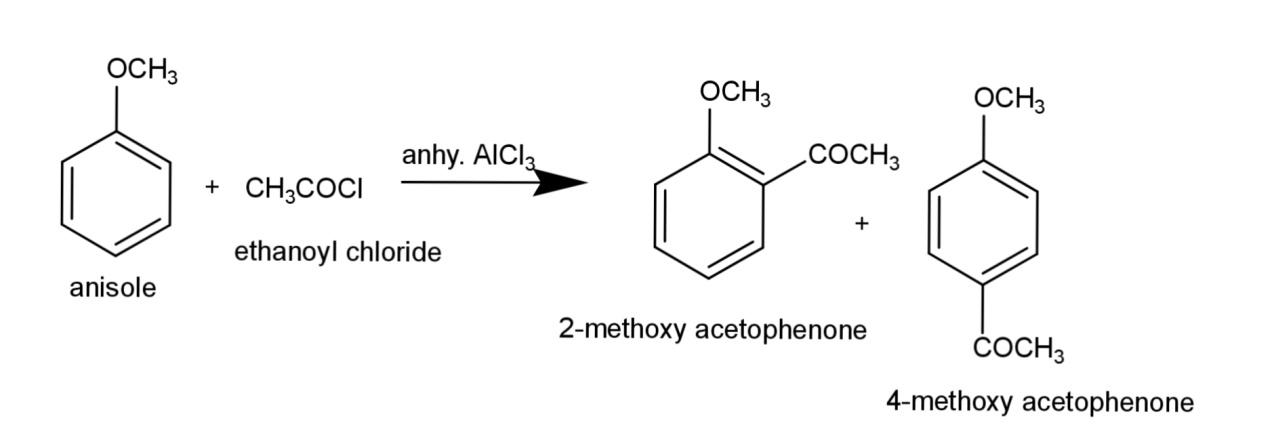
Hence, the reaction for alkylation, nitration, and acetylation of anisole are as above.
Note:
Friedal craft reaction is an important reaction. It uses anhydrous aluminum chloride that acts as a Lewis acid and is used to generate the electrophile that is different in terms of alkylation and acetylation. Aromatic ethers like anisole can be prepared by Williamson synthesis that is a reaction between alkoxides and ethers.
Complete answer:
Anisole is an aromatic compound that has a benzene ring and a $\left( -OC{{H}_{3}} \right)$group at the para position. It is a type of aromatic ether that undergoes electrophilic substitution reaction. The substituted group gets attached at the ortho and para positions as aromatic ethers are ortho – para directing due to the lone pair in conjugation with the ring so ortho – para positions acquire partial negative charges.
a)Alkylation of anisole consist of Friedal craft alkylation, where alkyl halide and anhydrous aluminum chloride is used, the equation for the reaction is:

b) Nitration of anisole involves the reaction of anisole with the nitrating mixture that consist of concentrated sulfuric acid and nitric acid, that results in substitution of nitro group and the reaction is:

c) Acetylation of anisole consists of substitution of the acetyl group on anisole, the reaction involves Friedal craft acetylation that involves ethanoyl chloride in presence of anhydrous aluminum chloride, the reaction equation is:

Hence, the reaction for alkylation, nitration, and acetylation of anisole are as above.
Note:
Friedal craft reaction is an important reaction. It uses anhydrous aluminum chloride that acts as a Lewis acid and is used to generate the electrophile that is different in terms of alkylation and acetylation. Aromatic ethers like anisole can be prepared by Williamson synthesis that is a reaction between alkoxides and ethers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




