
Write condensed formula and bond line formulae for the following structures.
a.
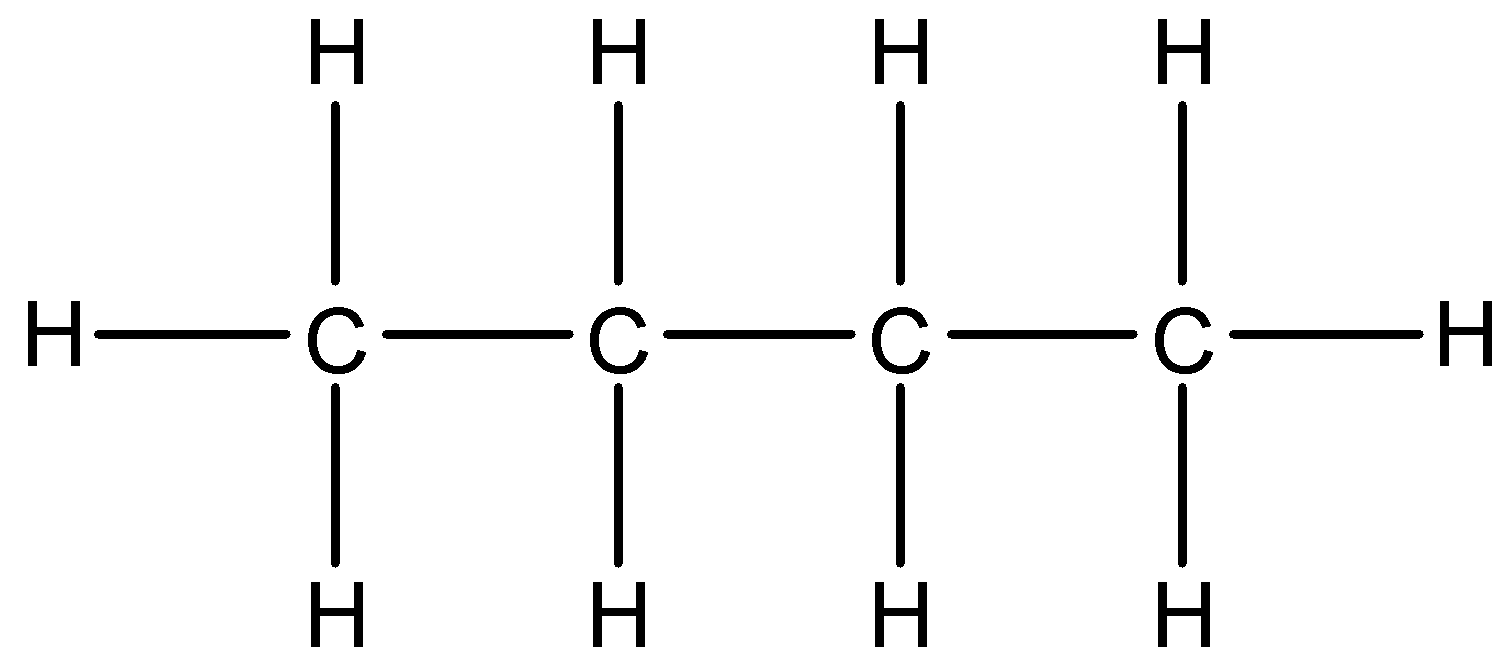
b.
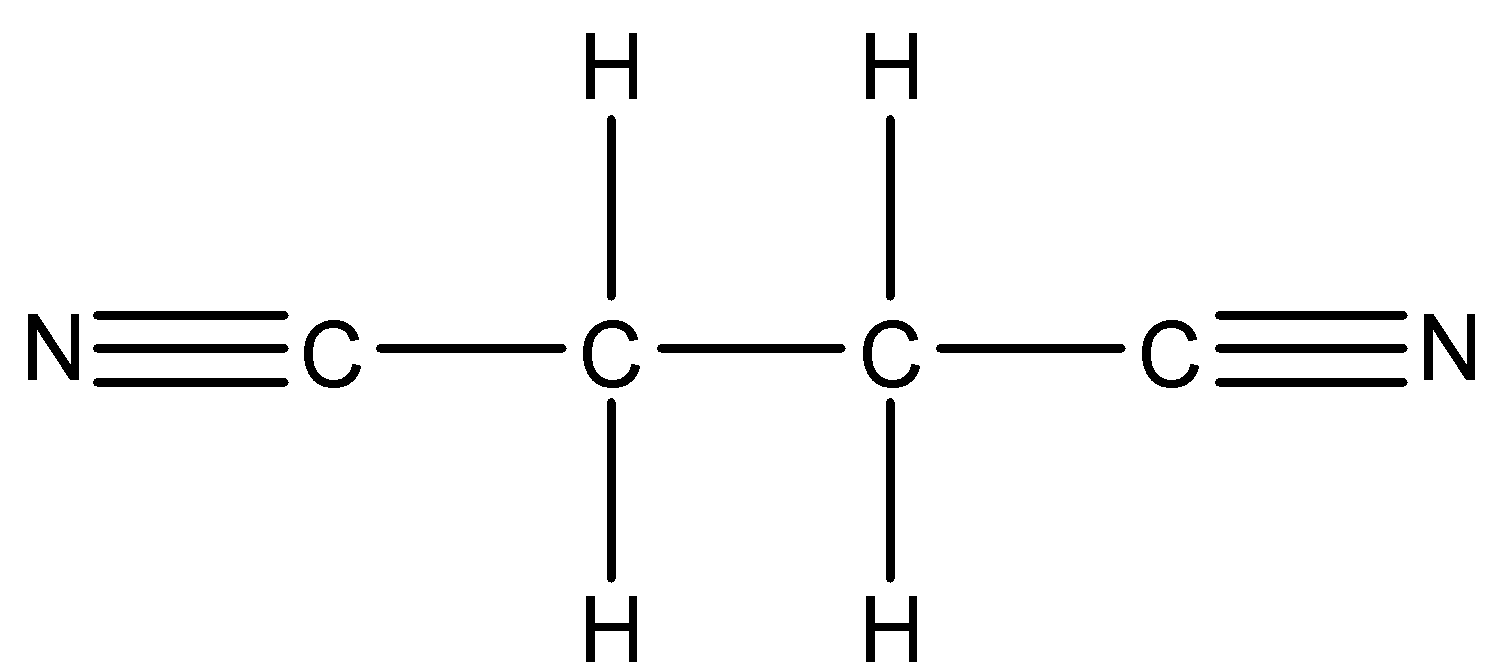
c.
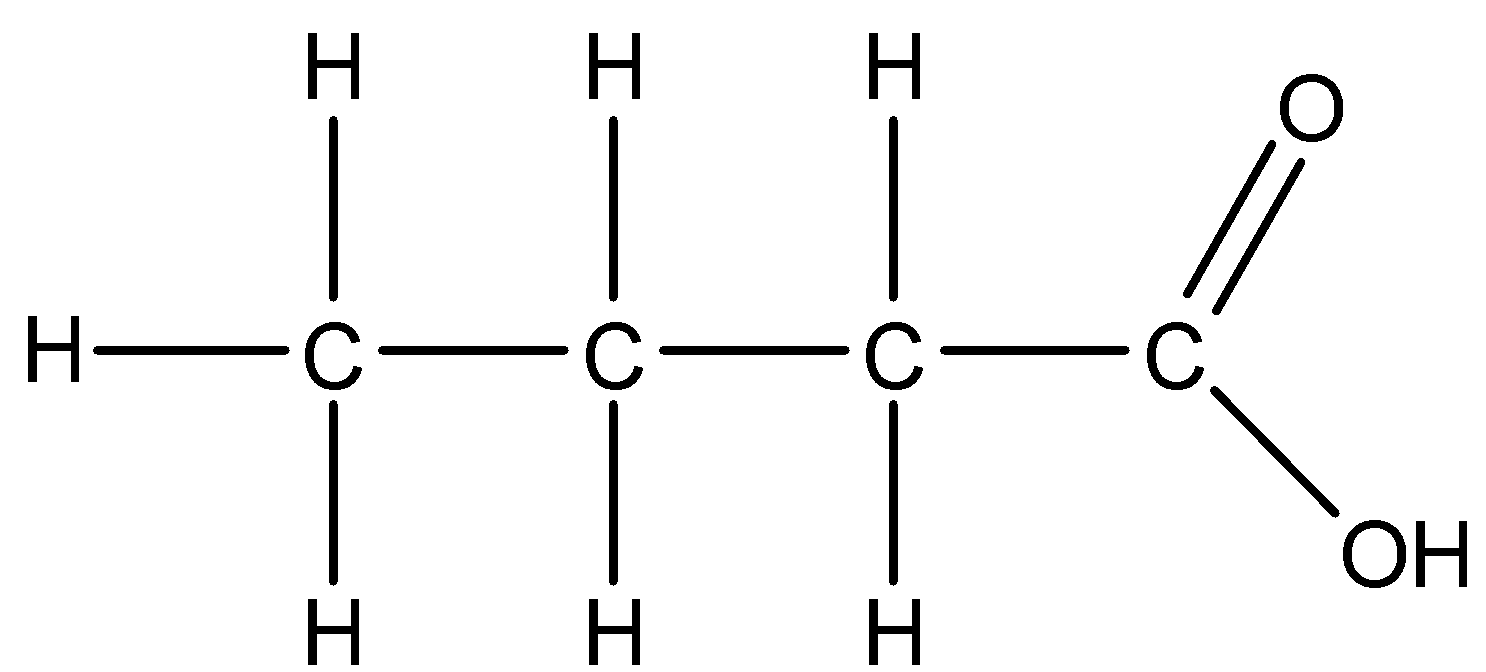



Answer
569.1k+ views
Hint: The chemical formula in which there is no presence of sigma or pi bonds is called condensed formula. The bond line formula is nothing but we have to represent the total structure of the compound without using the carbon symbol.
Complete Solution :
- In the question it is given that to write the condensed formula and bond line formulae for the given compounds.
- The first given structure is as follows.
a.
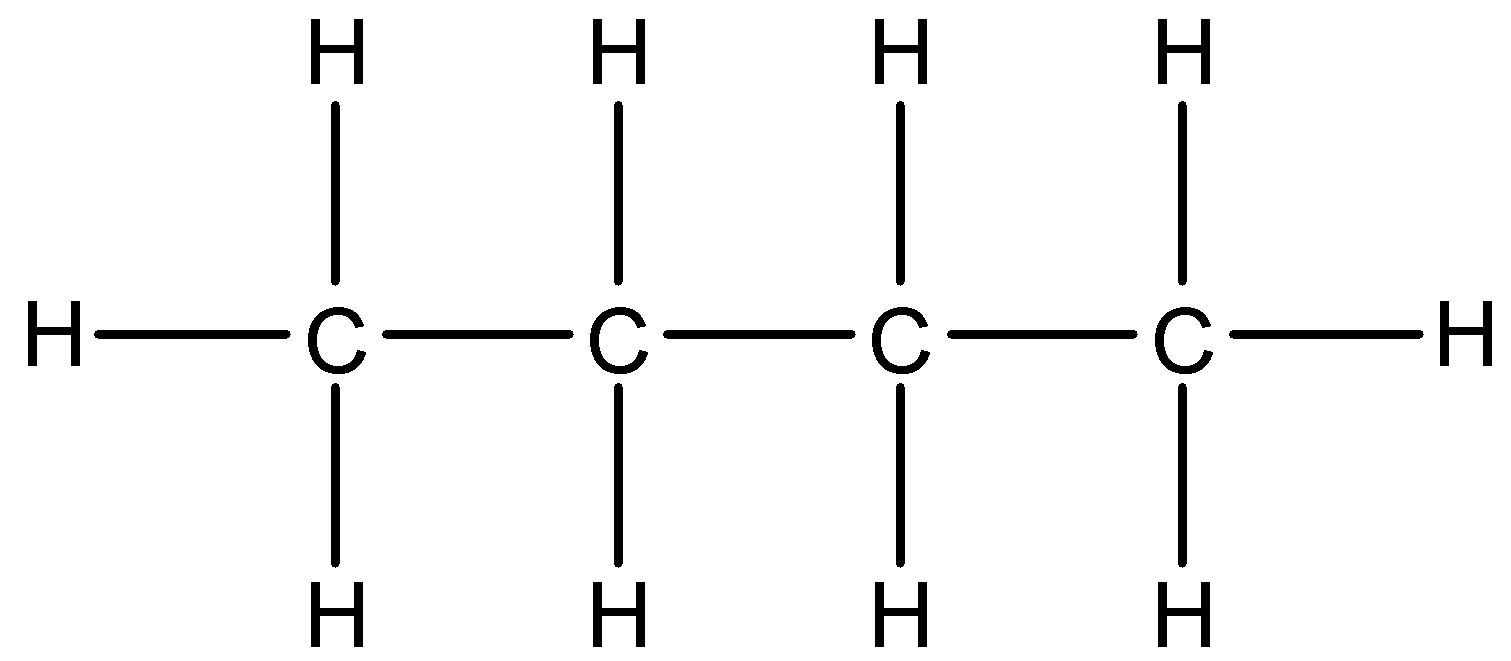
- The condensed formula of the structure ‘a’ is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$ .
- The bond line formula of the structure ‘a’ is as follows.
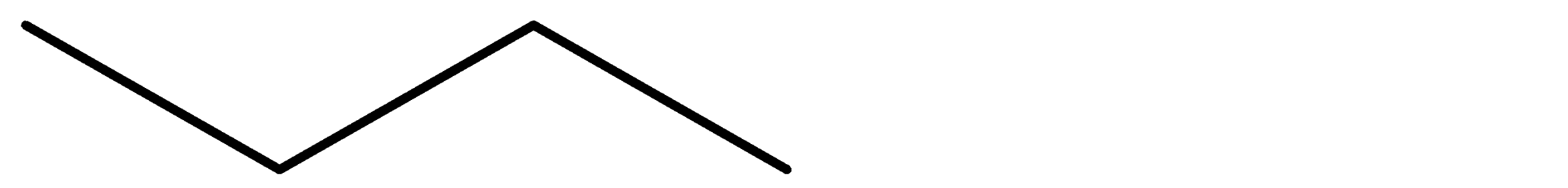
- The name of the structure ‘a’ is butane.
- The second given structure is as follows.
b.

- The condensed formula of the structure ‘b’ is $CNC{{H}_{2}}C{{H}_{2}}CN$ or ${{C}_{4}}{{H}_{4}}{{N}_{2}}$ .
- The bond line formula of the structure ‘b’ is as follows.
- The name of the structure b is butanonitrile.

- The third given structure is as follows.
c.

- The condensed formula of the structure ‘c’ is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH$ .
- The bond line formula of the structure ‘c’ is as follows.

- The name of the structure c is butanoic acid.
Note: Condensed formula gives the idea about the number of carbons, number of hydrogens, number of oxygen atoms, and number of nitrogen atoms present in the compound very easily. By using bond line formulas we can easily represent the structures of the organic compounds very easily.
Complete Solution :
- In the question it is given that to write the condensed formula and bond line formulae for the given compounds.
- The first given structure is as follows.
a.

- The condensed formula of the structure ‘a’ is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$ .
- The bond line formula of the structure ‘a’ is as follows.

- The name of the structure ‘a’ is butane.
- The second given structure is as follows.
b.

- The condensed formula of the structure ‘b’ is $CNC{{H}_{2}}C{{H}_{2}}CN$ or ${{C}_{4}}{{H}_{4}}{{N}_{2}}$ .
- The bond line formula of the structure ‘b’ is as follows.
- The name of the structure b is butanonitrile.

- The third given structure is as follows.
c.

- The condensed formula of the structure ‘c’ is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH$ .
- The bond line formula of the structure ‘c’ is as follows.

- The name of the structure c is butanoic acid.
Note: Condensed formula gives the idea about the number of carbons, number of hydrogens, number of oxygen atoms, and number of nitrogen atoms present in the compound very easily. By using bond line formulas we can easily represent the structures of the organic compounds very easily.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




