
Write chemical reactions to prepare the following polymers:
(A) Teflon
(B) Nylon-6
(C) Dextron
Answer
568.8k+ views
Hint: A molecule that can react with other molecules to form very large molecules having high molecular weights is known as a monomer. The high molecular weight molecules formed are known as polymers. The monomer is a repeating unit of polymer.
Complete Solution :
The reaction to prepare Teflon polymer is as follows:The process in which small molecules i.e. monomers combine chemically to produce a large chain-like molecule called a polymer.
We are given three polymers Teflon, nylon-6 and dextran.
(1) Teflon polymer can be prepared by the polymerization of monomer tetrafluoroethylene.
The monomer used to prepare Teflon polymer is tetrafluoroethylene.
The reaction to prepare Teflon polymer is as follows:
$n{\text{C}}{{\text{F}}_2} = {\text{C}}{{\text{F}}_2}\xrightarrow{{{\text{Polymerisation}}}}{\left[ { - {\text{C}}{{\text{F}}_2} = {\text{C}}{{\text{F}}_2} - } \right]_n}$
Teflon is a fluorocarbon solid. It contains fluorine and carbon. Teflon is hydrophobic in nature.
(2) Nylon-6 polymer can be prepared by the ring opening polymerization of monomer caprolactam.
The monomer used to prepare Nylon-6 polymer is caprolactam.
The reaction to prepare Nylon-6 polymer is as follows:
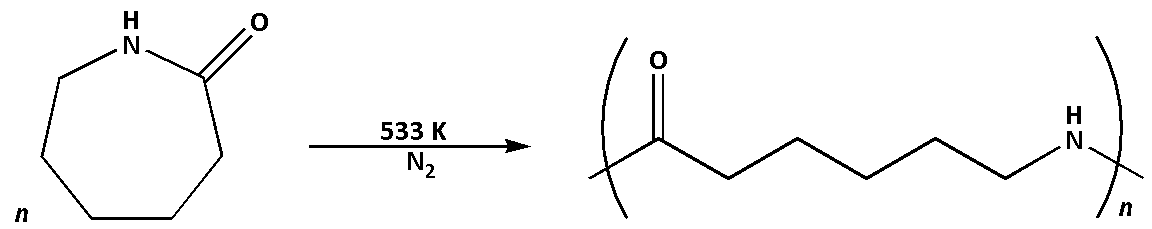
Nylon-6 is also known as polycaprolactam. Nylon-6 has a high strength and is very tough. Nylon-6 is easy to price.
(3) Dextron polymer can be prepared by an additional polymerization. Dextron is prepared by addition polymerisation of glycolic acid and lactic acid.
The monomers used to prepare Dextran polymers are glycolic acid and lactic acid.
The reaction to prepare Dextron polymer is as follows:
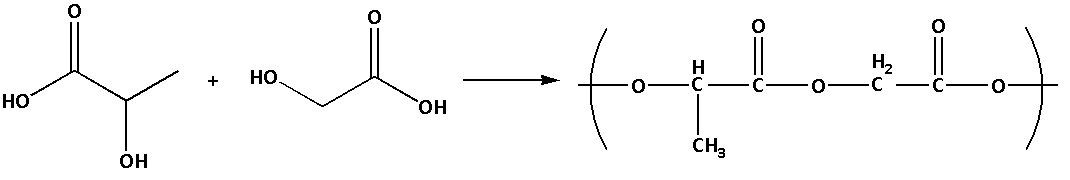
Dextron is a polymer of poly acetic acid and polyglycolic acid. The polymer Dextron has ester linkage. Dextron is used as a friction modifier.
Note: If Teflon is over heated it releases mildly toxic fumes. Do not confuse Nylon-6 with Nylon-6,6 which is prepared from adipic acid and hexamethylenediamine. Dextron is used in lubricants to reduce surface friction.
Complete Solution :
The reaction to prepare Teflon polymer is as follows:The process in which small molecules i.e. monomers combine chemically to produce a large chain-like molecule called a polymer.
We are given three polymers Teflon, nylon-6 and dextran.
(1) Teflon polymer can be prepared by the polymerization of monomer tetrafluoroethylene.
The monomer used to prepare Teflon polymer is tetrafluoroethylene.
The reaction to prepare Teflon polymer is as follows:
$n{\text{C}}{{\text{F}}_2} = {\text{C}}{{\text{F}}_2}\xrightarrow{{{\text{Polymerisation}}}}{\left[ { - {\text{C}}{{\text{F}}_2} = {\text{C}}{{\text{F}}_2} - } \right]_n}$
Teflon is a fluorocarbon solid. It contains fluorine and carbon. Teflon is hydrophobic in nature.
(2) Nylon-6 polymer can be prepared by the ring opening polymerization of monomer caprolactam.
The monomer used to prepare Nylon-6 polymer is caprolactam.
The reaction to prepare Nylon-6 polymer is as follows:

Nylon-6 is also known as polycaprolactam. Nylon-6 has a high strength and is very tough. Nylon-6 is easy to price.
(3) Dextron polymer can be prepared by an additional polymerization. Dextron is prepared by addition polymerisation of glycolic acid and lactic acid.
The monomers used to prepare Dextran polymers are glycolic acid and lactic acid.
The reaction to prepare Dextron polymer is as follows:

Dextron is a polymer of poly acetic acid and polyglycolic acid. The polymer Dextron has ester linkage. Dextron is used as a friction modifier.
Note: If Teflon is over heated it releases mildly toxic fumes. Do not confuse Nylon-6 with Nylon-6,6 which is prepared from adipic acid and hexamethylenediamine. Dextron is used in lubricants to reduce surface friction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




