
Williamson’s synthesis uses _ _ _ _ _ _ mechanism.
(A)- ${{S}_{N}}1$
(B)- ${{S}_{N}}2$
(C)- either ${{S}_{N}}1$ or ${{S}_{N}}2$
(D)- none
Answer
590.1k+ views
Hint: Williamson’s synthesis is one of the best processes used for the synthesis of both symmetrical and unsymmetrical ethers. Alkyl halides react with sodium alkoxide to form ethers. General reaction for the formation of ether by Williamson’s synthesis can be written as
\[R{{O}^{-}}N{{a}^{-}}+{R}'-X\to R-O-{R}'+N{{a}^{+}}{{X}^{-}}\]
Complete step by step answer:
Williamson’s ether synthesis involves the preparation of ethers by nucleophilic substitution reaction.
In alkyl halides, carbon halogen bond is polar due to higher electronegativity of the halogen atoms. As a result, positive charge develops on the carbon attached to the halogen atom making it somewhat electrophilic in nature.
\[{{R}^{\delta +}}-{{X}^{\delta -}}\]
Alkoxide ion acts as a nucleophile and substitutes the halide ion from an alkyl halide. The reaction occurs by bimolecular nucleophilic substitution, i.e. ${{S}_{N}}2$ mechanism.
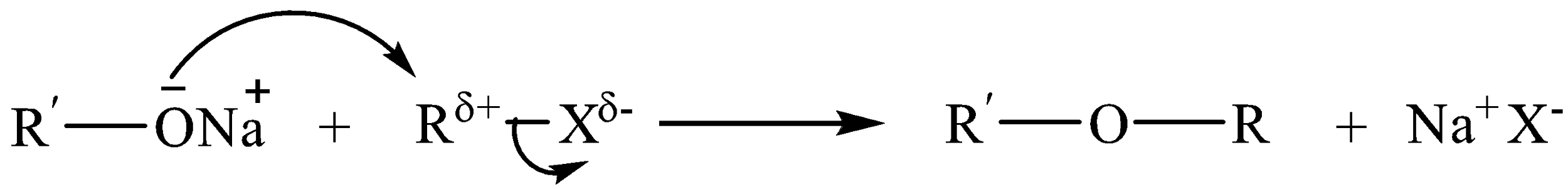
Let us have a look at some of the examples.
Preparation of symmetrical ethers
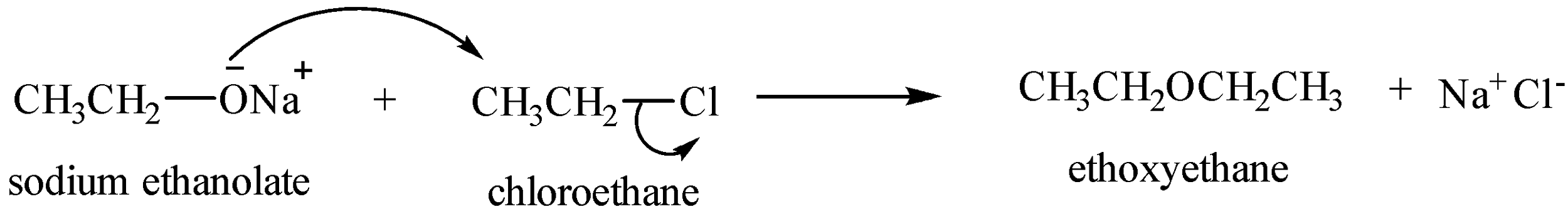
Preparation of unsymmetrical alkenes
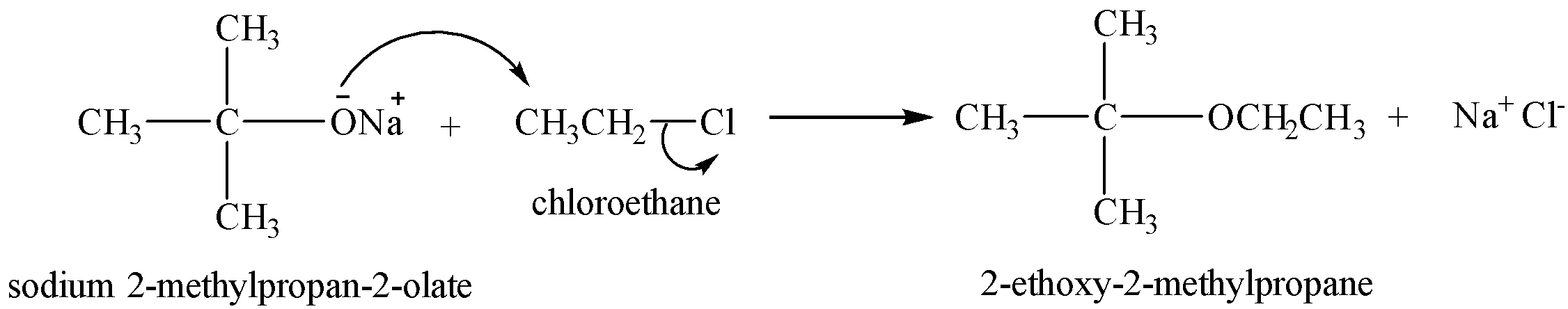
For the preparation of unsymmetrical ethers, primary alkyl halides are taken.
The reaction proceeds via ${{S}_{N}}2$ mechanism, where the nucleophile attacks the carbon from the back side, so if secondary or tertiary alkyl halides are used, the nucleophile attack is hindered by the alkyl groups on the ${{2}^{o}}$ and ${{3}^{o}}$ carbons.
Therefore, in case of secondary and tertiary alkyl halide, the alkoxide ion behaves as a base and abstracts the $\beta $-hydrogens, thus resulting in the formation of an alkene.
Since the reaction follows ${{S}_{N}}2$ mechanism, the reactivity of alkyl halides in Williamson’s synthesis follows the same order as that in${{S}_{N}}2$ mechanism, i.e. ${{1}^{o}}>{{2}^{o}}>{{3}^{o}}$
So, the correct answer is “Option B”.
Note: Remember that ${{S}_{N}}2$ reaction is a one step reaction and bond breaking and making takes place simultaneously, hence no intermediate is formed. Due to the backside attack of the nucleophile on alkyl halide the configuration of the product formed is reversed.
\[R{{O}^{-}}N{{a}^{-}}+{R}'-X\to R-O-{R}'+N{{a}^{+}}{{X}^{-}}\]
Complete step by step answer:
Williamson’s ether synthesis involves the preparation of ethers by nucleophilic substitution reaction.
In alkyl halides, carbon halogen bond is polar due to higher electronegativity of the halogen atoms. As a result, positive charge develops on the carbon attached to the halogen atom making it somewhat electrophilic in nature.
\[{{R}^{\delta +}}-{{X}^{\delta -}}\]
Alkoxide ion acts as a nucleophile and substitutes the halide ion from an alkyl halide. The reaction occurs by bimolecular nucleophilic substitution, i.e. ${{S}_{N}}2$ mechanism.

Let us have a look at some of the examples.
Preparation of symmetrical ethers
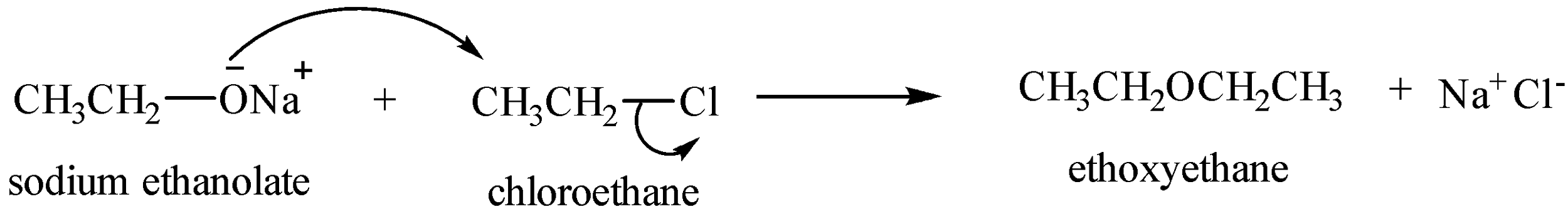
Preparation of unsymmetrical alkenes

For the preparation of unsymmetrical ethers, primary alkyl halides are taken.
The reaction proceeds via ${{S}_{N}}2$ mechanism, where the nucleophile attacks the carbon from the back side, so if secondary or tertiary alkyl halides are used, the nucleophile attack is hindered by the alkyl groups on the ${{2}^{o}}$ and ${{3}^{o}}$ carbons.
Therefore, in case of secondary and tertiary alkyl halide, the alkoxide ion behaves as a base and abstracts the $\beta $-hydrogens, thus resulting in the formation of an alkene.
Since the reaction follows ${{S}_{N}}2$ mechanism, the reactivity of alkyl halides in Williamson’s synthesis follows the same order as that in${{S}_{N}}2$ mechanism, i.e. ${{1}^{o}}>{{2}^{o}}>{{3}^{o}}$
So, the correct answer is “Option B”.
Note: Remember that ${{S}_{N}}2$ reaction is a one step reaction and bond breaking and making takes place simultaneously, hence no intermediate is formed. Due to the backside attack of the nucleophile on alkyl halide the configuration of the product formed is reversed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




