
Which one of the following properties is not shown by NO?
(A) It combines with oxygen to form nitrogen dioxide.
(B) Its bond order is 2.5.
(C) It is diamagnetic in gaseous state.
(D) It is a neutral oxide.
Answer
579.3k+ views
Hint: Think about the properties of nitric oxide. Write the electronic configuration of nitrogen and oxygen and then try to draw the molecular orbital diagram of nitric oxide, NO. Then calculate the bond order of NO and check its magnetism. Write the reaction of NO with oxygen and see if NO dissolves in water and its reactivity with acids and bases and then select the correct option.
Complete step by step answer:
- NO is nitric oxide. NO reacts with oxygen to form nitrogen dioxide. This reaction is used to prepare nitrogen dioxide and is given as, $2NO+{{O}_{2}}\to 2N{{O}_{2}}$
- Nitrogen has the atomic number 7 and oxygen has the atomic number 8. Their electronic configurations are given as,
\[{}^{7}N=1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}\]
\[{}^{8}O=1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
- Molecular orbital diagram of nitric oxide can be represented as,
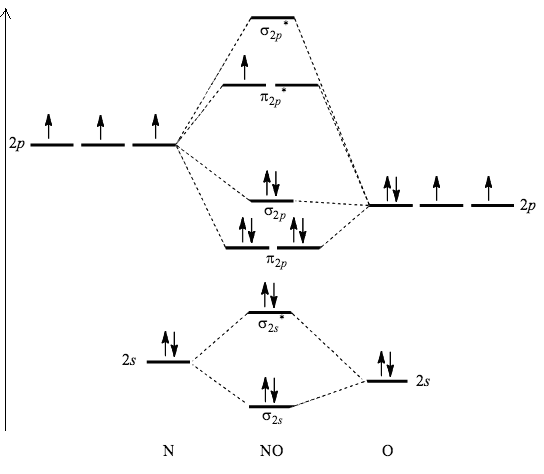
- Now, we can see that an anti-bonding orbital of NO has one unpaired electron and so, it is paramagnetic in nature.
- The bond order of NO is calculated as $\dfrac{8-3}{2}=\dfrac{5}{2}=2.5$
- Nitric oxide dissolves in water and doesn’t dissolve in acids and bases. Therefore, NO is a neutral oxide.
So, the correct answer is “Option D”.
Note: Remember nitric oxide is a neutral oxide and it reacts with oxygen to form nitrogen dioxide. Nitric oxide is paramagnetic in nature due to the presence of an unpaired electron. Nitric oxide will be resonance stabilized and hence have a partial triple bond character and therefore, its bond order 2.5 is correct.
Complete step by step answer:
- NO is nitric oxide. NO reacts with oxygen to form nitrogen dioxide. This reaction is used to prepare nitrogen dioxide and is given as, $2NO+{{O}_{2}}\to 2N{{O}_{2}}$
- Nitrogen has the atomic number 7 and oxygen has the atomic number 8. Their electronic configurations are given as,
\[{}^{7}N=1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}\]
\[{}^{8}O=1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
- Molecular orbital diagram of nitric oxide can be represented as,

- Now, we can see that an anti-bonding orbital of NO has one unpaired electron and so, it is paramagnetic in nature.
- The bond order of NO is calculated as $\dfrac{8-3}{2}=\dfrac{5}{2}=2.5$
- Nitric oxide dissolves in water and doesn’t dissolve in acids and bases. Therefore, NO is a neutral oxide.
So, the correct answer is “Option D”.
Note: Remember nitric oxide is a neutral oxide and it reacts with oxygen to form nitrogen dioxide. Nitric oxide is paramagnetic in nature due to the presence of an unpaired electron. Nitric oxide will be resonance stabilized and hence have a partial triple bond character and therefore, its bond order 2.5 is correct.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




