
Which one of the following is not expected to undergo iodoform reaction $ ? $
(A) Propan $ - 2 - $ ol
(B) $ 1 - $ Phenyl ethanol
(C) $ 2 - $ Butanol
(D) Ethanol
(E) Diphenyl methanol
Answer
528.6k+ views
Hint :Iodoform is an iodine containing organic compound with molecular formula $ CH{I_3} $ . It is a crystalline solid that has a characteristic pale yellow colour and distinct odour. Iodoform test is used to determine the presence of $ C{H_3}CO $ or $ C{H_3}CH(OH) $ groups in the given organic compound. It is a test given by certain aldehydes, ketones, and secondary alcohols.
Complete Step By Step Answer:
Iodoform test is given by aldehydes, ketones, secondary alcohols that have an alpha methyl group attached to the carbon containing functional group. It tests the presence of $ C{H_3}CO $ and $ C{H_3}CH(OH) $ . When iodine reacts with a base (NaOH) and the carbonyl group of the organic compound, it forms a yellow precipitate which is iodoform. Below is an example of an iodoform test.
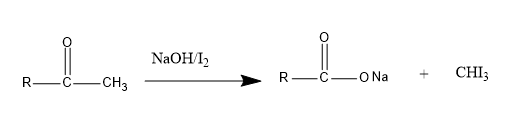
Now, let us examine the given compounds.
(A)
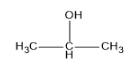
It is a secondary alcohol and undergoes iodoform test since it contains $ C{H_3}CH(OH) $ group.
(B)
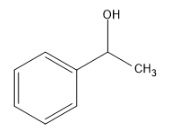
Compound contains the $ C{H_3}CH(OH) $ group and therefore gives positive results for the iodoform test.
(C)
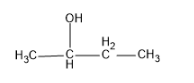
$ 2 - $ Butanol also contains $ C{H_3}CH(OH) $ group and hence a yellow precipitate of iodoform is formed.
(D)
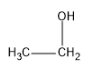
Ethanol is the only primary alcohol that gives an iodoform test.
(E)
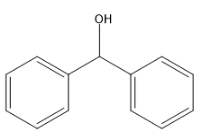
It does not contain any alpha methyl group. Iodoform is not formed when the given compound reacts with NaOH and $ {I_2} $ . There is no $ C{H_3}CO $ or $ C{H_3}CH(OH) $ group.
Therefore, the right option is (E) Diphenyl methanol.
Note :
Iodoform also known as triiodomethane or carbon triiodide has a distinct odour that is similar to chloroform. It has antimicrobial properties and is used as disinfectant. It is also used as an antiseptic.
Acetaldehyde is the only aldehyde that gives positive iodoform test.
Iodoform test is an important reaction used for the production of iodoform.
Complete Step By Step Answer:
Iodoform test is given by aldehydes, ketones, secondary alcohols that have an alpha methyl group attached to the carbon containing functional group. It tests the presence of $ C{H_3}CO $ and $ C{H_3}CH(OH) $ . When iodine reacts with a base (NaOH) and the carbonyl group of the organic compound, it forms a yellow precipitate which is iodoform. Below is an example of an iodoform test.

Now, let us examine the given compounds.
(A)

It is a secondary alcohol and undergoes iodoform test since it contains $ C{H_3}CH(OH) $ group.
(B)

Compound contains the $ C{H_3}CH(OH) $ group and therefore gives positive results for the iodoform test.
(C)

$ 2 - $ Butanol also contains $ C{H_3}CH(OH) $ group and hence a yellow precipitate of iodoform is formed.
(D)

Ethanol is the only primary alcohol that gives an iodoform test.
(E)

It does not contain any alpha methyl group. Iodoform is not formed when the given compound reacts with NaOH and $ {I_2} $ . There is no $ C{H_3}CO $ or $ C{H_3}CH(OH) $ group.
Therefore, the right option is (E) Diphenyl methanol.
Note :
Iodoform also known as triiodomethane or carbon triiodide has a distinct odour that is similar to chloroform. It has antimicrobial properties and is used as disinfectant. It is also used as an antiseptic.
Acetaldehyde is the only aldehyde that gives positive iodoform test.
Iodoform test is an important reaction used for the production of iodoform.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




