
Which one of the following compounds will give in the presence of peroxide a product different from that obtained in the absence of peroxide.
A. 1-butene,$HCl$
B. 1-butene, $HBr$
C. 2-butene, $HCl$
D. 2-butene, $HBr$
Answer
573.9k+ views
Hint: The addition of halogen halides in alkene gives alkyl halide in general. When the alkene is symmetrical we get only one theoretical product whereas in case of unsymmetrical alkenes two different rules are available, i.e.
Markovnikov’s rule
Antimarkovnikov’s rule
Complete step by step answer:
As per the above question, we have 2-butene which is a symmetrical alkene involved, so we will get only one theoretical product. When $HCl$ reacts with 2-butene as in Option C, the formation of 2-chloro butane is observed. The chemical reaction for the above is given as:
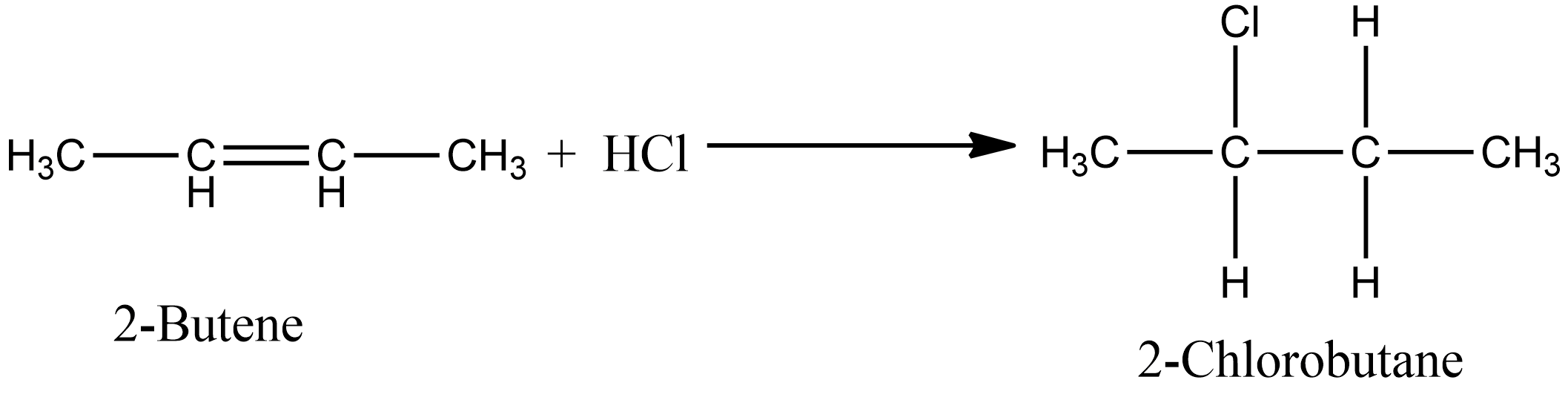
Similarly, in the case of option D i.e. during the addition of $HBr$ in 2- butene, there is also the formation of one product i.e. 2-Bromo butane. The chemical reaction for the same is given as:
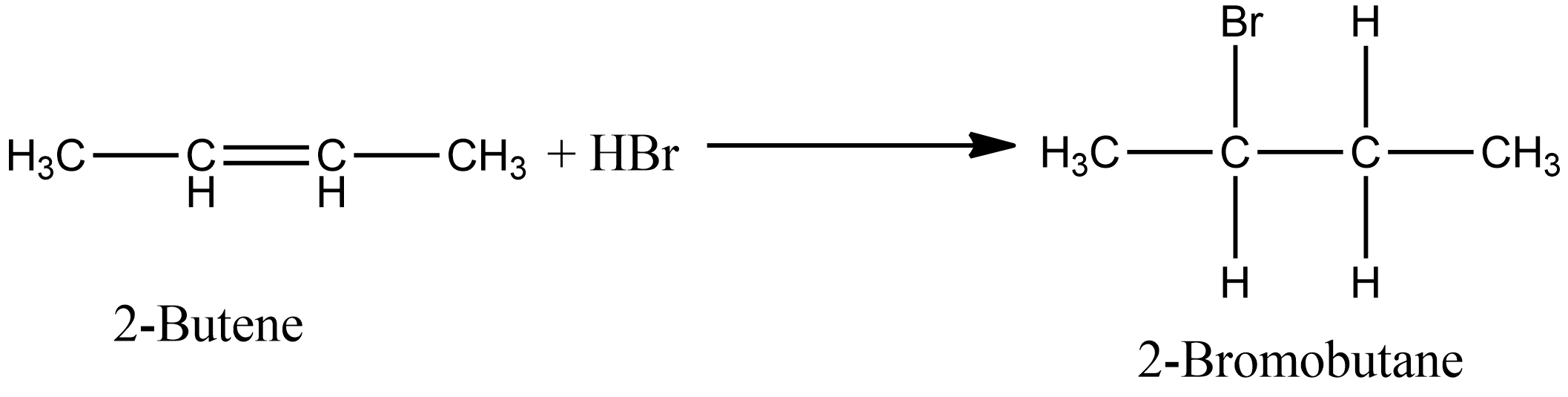
Options A and B involve the addition of unsymmetrical alkenes, if the reaction proceeds in the presence of peroxide then antimarkovnikov’s rule applies else in absence of peroxide markovnikov’s rule apply. Option A involves the addition of $HCl$, so antimarkovnikov’s rule is not applicable. So according to markovnikov’s rule the product of the reaction is given as:
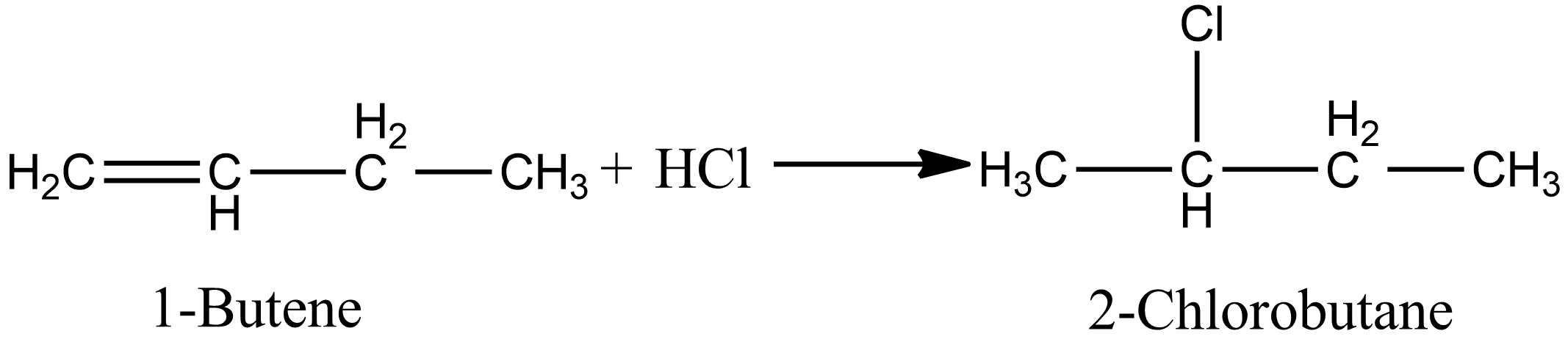
In the case of option B, i.e. during the ad
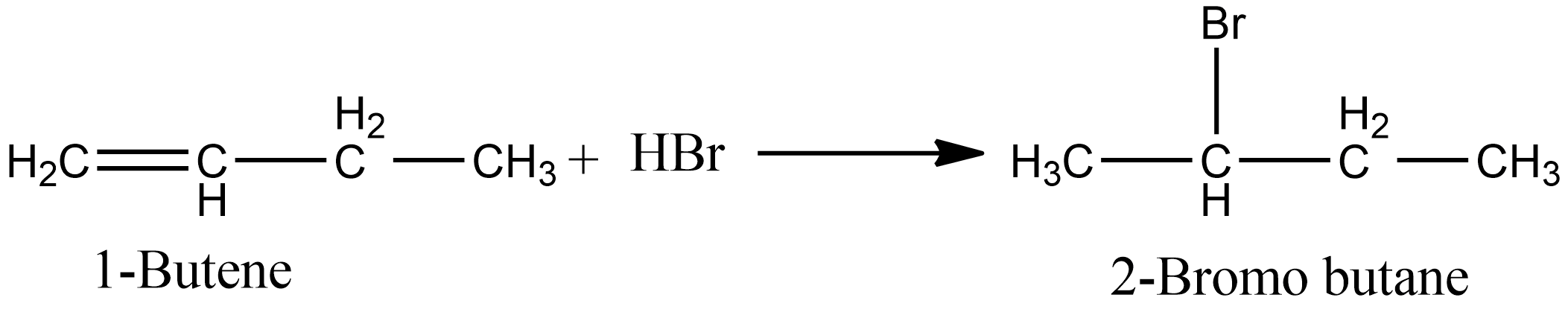 addition of $HBr$ in 1-butene, both markovnikov’s and anti markovnikov’s rule apply. When the reaction proceeds are per markovnikov’s, i.e. in absence of peroxide we obtain 2-Bromo butane. The reaction involved is shown below:
addition of $HBr$ in 1-butene, both markovnikov’s and anti markovnikov’s rule apply. When the reaction proceeds are per markovnikov’s, i.e. in absence of peroxide we obtain 2-Bromo butane. The reaction involved is shown below:
And in the presence of peroxide, i.e as per anti markovnikov’s, the product obtained is 1-Bromo butane. The reaction involved is shown below:
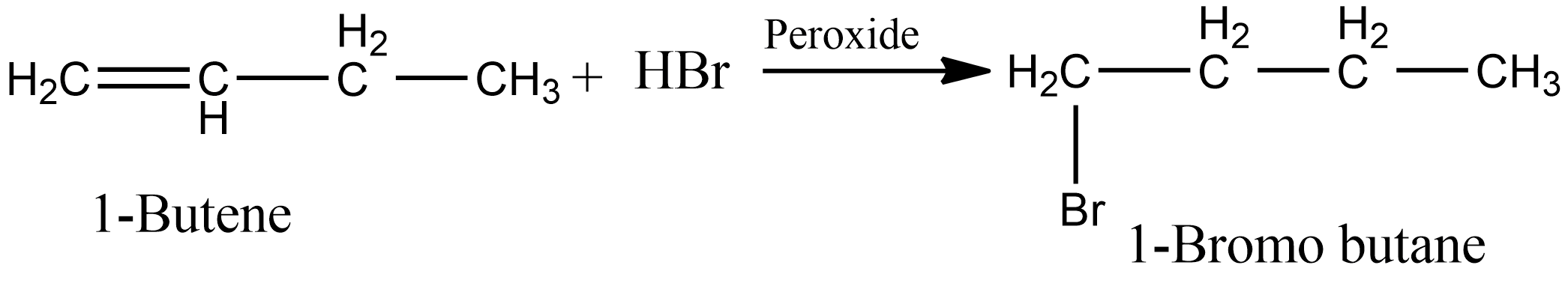
Hence we can say in case option B, the same product is obtained in the presence of peroxide as well as in the absence of peroxide.
So, the correct answer is Option B.
Note: Antimarkovnikov’ s rule is also known as the peroxide effect and is applicable only in case of $HBr$ because both the steps are exothermic but in the case of $HCl$ and $HF$ the second step involving the reaction of carbon, radicals are endothermic whereas in the case of $HI$ the first step involving the addition of iodine radicals to an alkene is endothermic.
Markovnikov’s rule
Antimarkovnikov’s rule
Complete step by step answer:
As per the above question, we have 2-butene which is a symmetrical alkene involved, so we will get only one theoretical product. When $HCl$ reacts with 2-butene as in Option C, the formation of 2-chloro butane is observed. The chemical reaction for the above is given as:

Similarly, in the case of option D i.e. during the addition of $HBr$ in 2- butene, there is also the formation of one product i.e. 2-Bromo butane. The chemical reaction for the same is given as:

Options A and B involve the addition of unsymmetrical alkenes, if the reaction proceeds in the presence of peroxide then antimarkovnikov’s rule applies else in absence of peroxide markovnikov’s rule apply. Option A involves the addition of $HCl$, so antimarkovnikov’s rule is not applicable. So according to markovnikov’s rule the product of the reaction is given as:
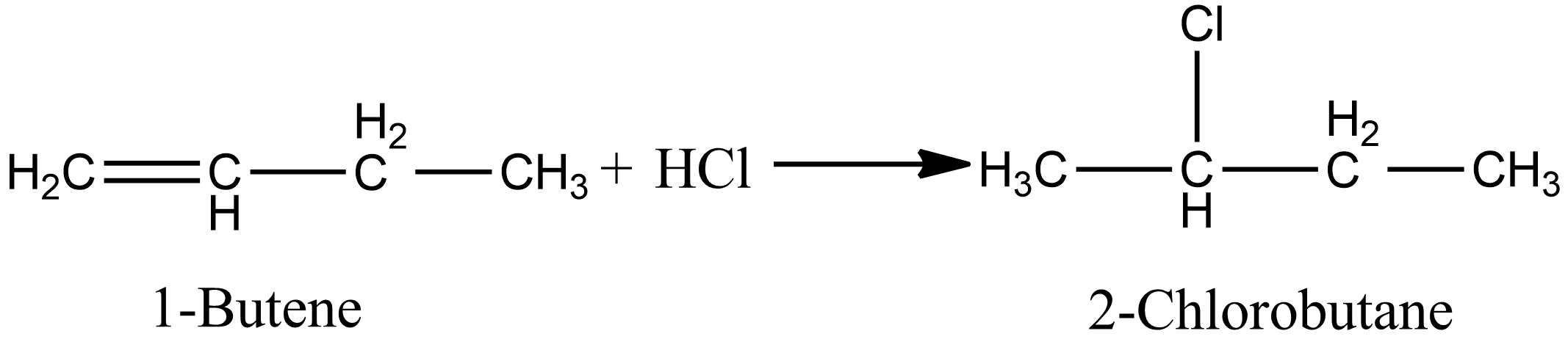
In the case of option B, i.e. during the ad
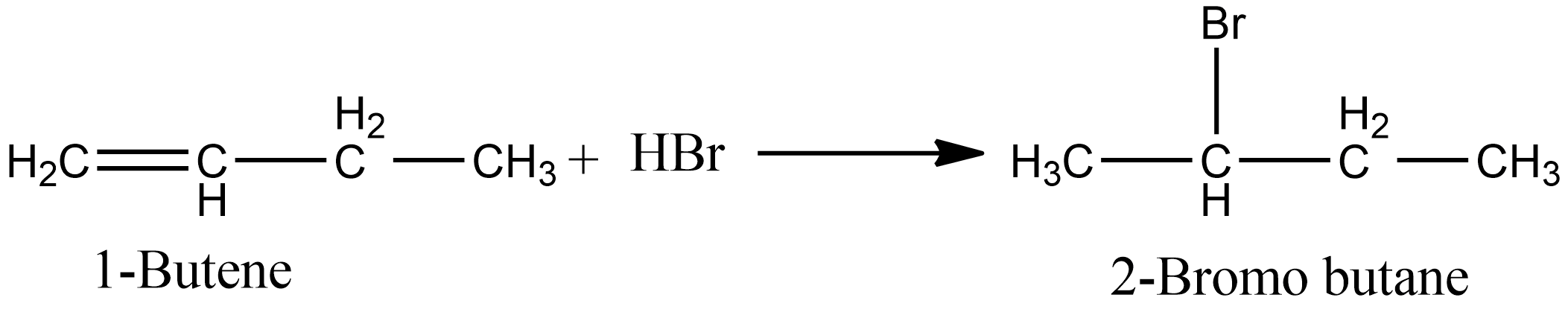
And in the presence of peroxide, i.e as per anti markovnikov’s, the product obtained is 1-Bromo butane. The reaction involved is shown below:
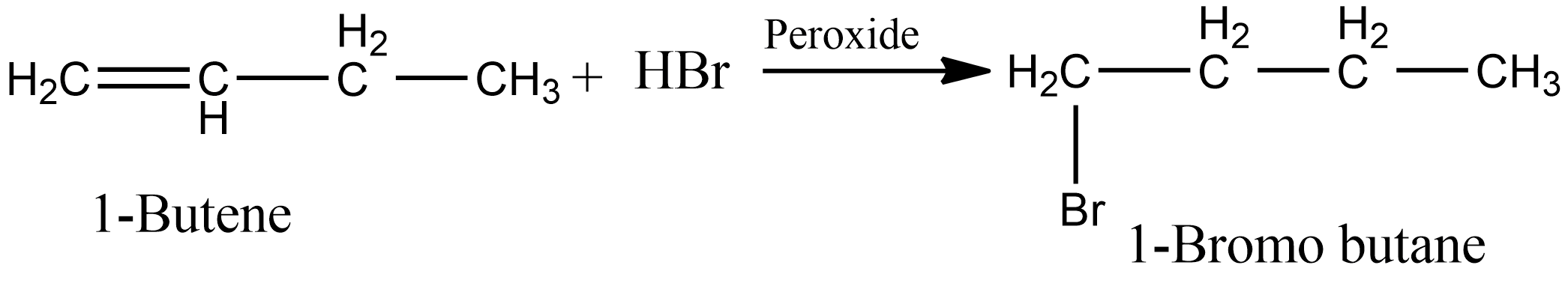
Hence we can say in case option B, the same product is obtained in the presence of peroxide as well as in the absence of peroxide.
So, the correct answer is Option B.
Note: Antimarkovnikov’ s rule is also known as the peroxide effect and is applicable only in case of $HBr$ because both the steps are exothermic but in the case of $HCl$ and $HF$ the second step involving the reaction of carbon, radicals are endothermic whereas in the case of $HI$ the first step involving the addition of iodine radicals to an alkene is endothermic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




