
Which of these has a triple covalent bond?
(A) $ P{H_3} $
(B) $ C{O_2} $
(C) $ AlC{l_3} $
(D) $ {C_2}{H_2} $
Answer
517.5k+ views
Hint : Covalent bonds are the strong bonds formed by the equal sharing of electrons between the two atoms. The electrons are known as sharing pairs or bonding pairs of electrons. Covalent bond is the stable bond formed by the proper amount of repulsive force and the attractive forces.
Complete Step By Step Answer:
As we know covalent bonds have equal sharing of electrons between the two atoms, so they are one of the strongest bonds.
Let’s see which among the given options contains the three covalent bonds.
So for three covalent bonds to occur in a molecule, that should contain one sigma bond, $ 1\sigma $ and two pi bonds $ 2\pi $ . This concludes that there should be the sharing of six electrons in total. So the triple covalent bond has high electron density around them.
So, in our given options alkene, $ {C_2}{H_2} $ is best suited to have a triple covalent bond consisting of one sigma $ 1\sigma $ and two pi bonds, $ 2\pi $ .
Structure is:
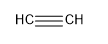
So, there is a sharing of six electrons, having high density of electrons around the covalent bond. Out of these three covalent bonds , one is sigma bond and two is pii bond.
Therefore, our correct option is D.
Note :
There are other types of bondings also which are ionic bonding and coordination bonding. In the ionic bonding, unlike covalent bonding, electrons are completely transferred to one atom. This bonding give rise to the ions. The atom that gains the electron is known as anion and the atom which loses the electrons, becomes positively charged and is known as cation.
Complete Step By Step Answer:
As we know covalent bonds have equal sharing of electrons between the two atoms, so they are one of the strongest bonds.
Let’s see which among the given options contains the three covalent bonds.
So for three covalent bonds to occur in a molecule, that should contain one sigma bond, $ 1\sigma $ and two pi bonds $ 2\pi $ . This concludes that there should be the sharing of six electrons in total. So the triple covalent bond has high electron density around them.
So, in our given options alkene, $ {C_2}{H_2} $ is best suited to have a triple covalent bond consisting of one sigma $ 1\sigma $ and two pi bonds, $ 2\pi $ .
Structure is:
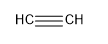
So, there is a sharing of six electrons, having high density of electrons around the covalent bond. Out of these three covalent bonds , one is sigma bond and two is pii bond.
Therefore, our correct option is D.
Note :
There are other types of bondings also which are ionic bonding and coordination bonding. In the ionic bonding, unlike covalent bonding, electrons are completely transferred to one atom. This bonding give rise to the ions. The atom that gains the electron is known as anion and the atom which loses the electrons, becomes positively charged and is known as cation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




