
Which of the molecules is trigonal bipyramidal?
A. $B{{F}_{3}}$
B. $C{{H}_{4}}$
C. $PC{{l}_{5}}$
D. $S{{F}_{6}}$
Answer
561.6k+ views
Hint: The number of bonds, lone pair of electrons, and the hybridization of the central atom explain the shape of the molecules. If there is a presence of a lone pair of electrons on the central atom the shape of the molecule is going to change.
Complete step by step answer:
- In the question, it is asked which molecules have the shape of trigonal bipyramidal among the given options.
- First, we should know the number of lone pairs on the central atom, the number of bonds, and the hybridization of the given molecules to find the shape of the given molecules.
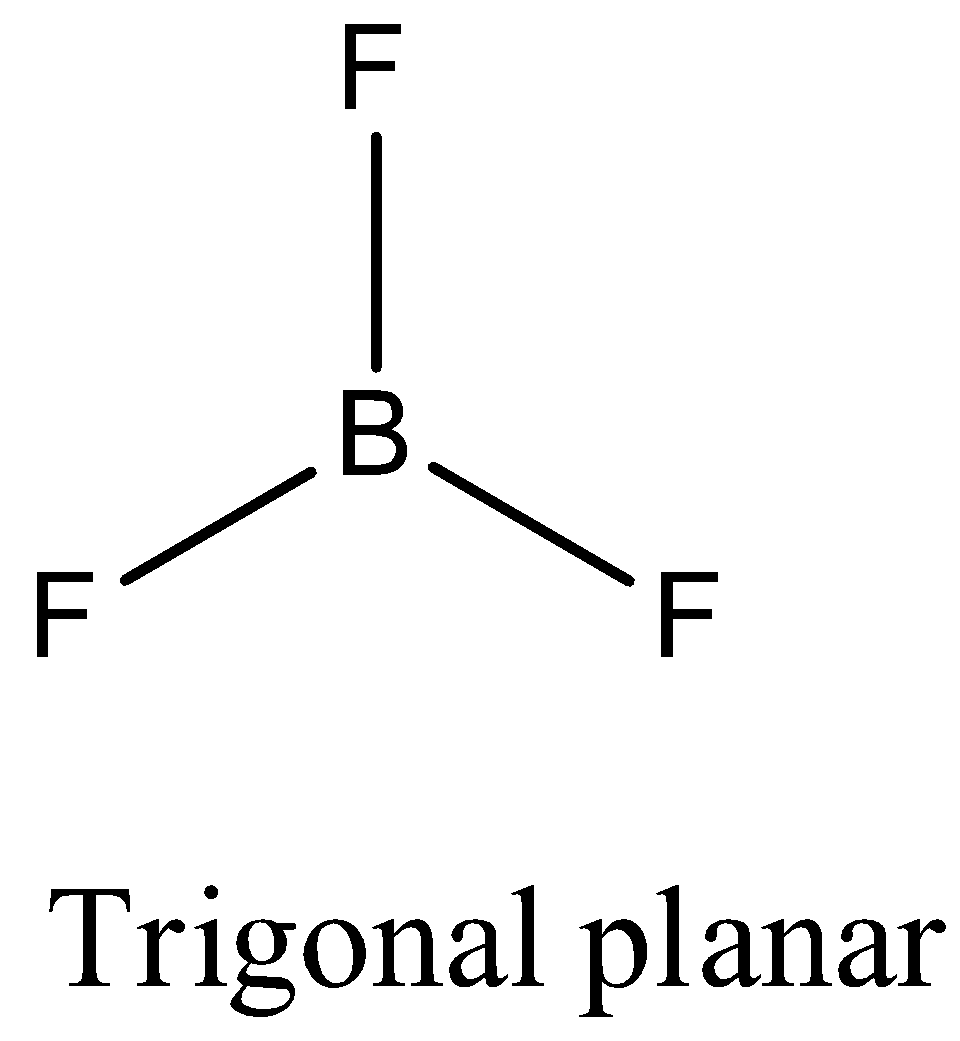
- Coming to option A, $B{{F}_{3}}$. The boron atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is $s{{p}^{2}}$ and the number of bonds is three then the structure of $B{{F}_{3}}$ is trigonal planar and it is as follows.
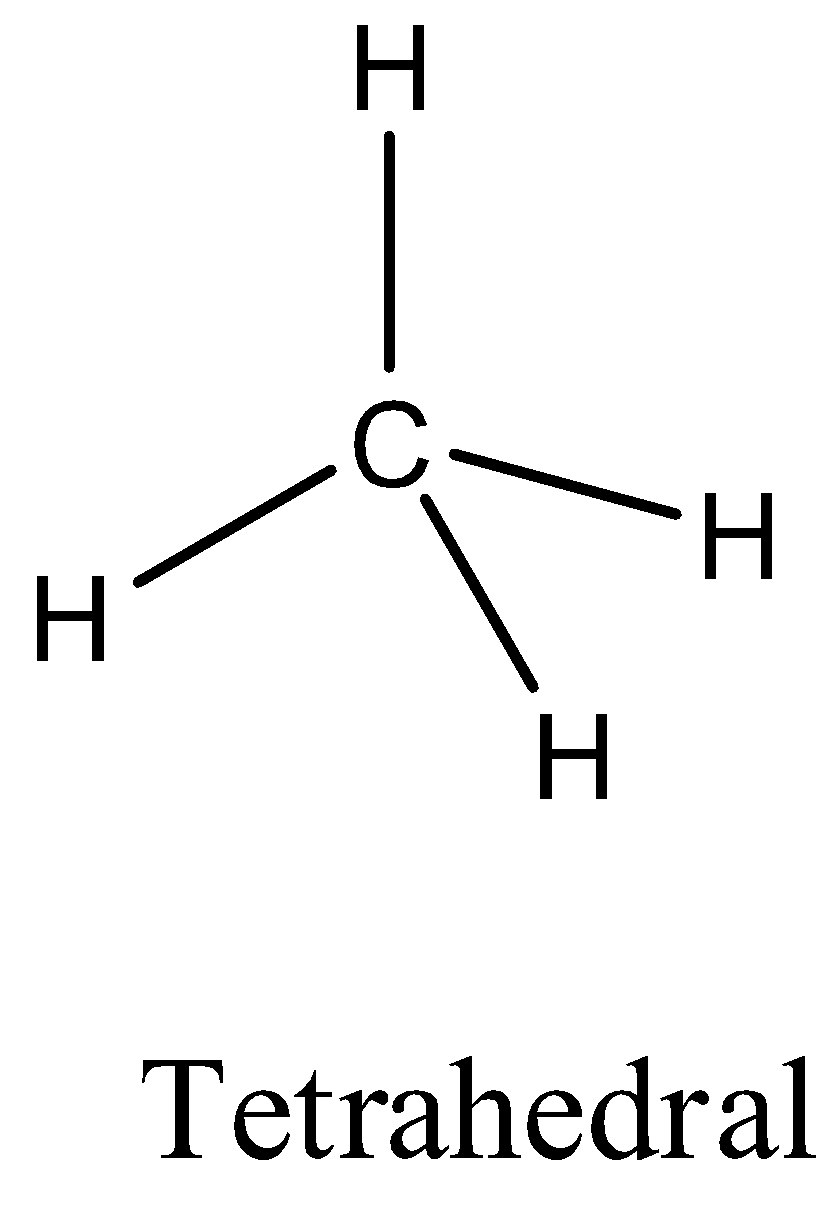
- Coming to option B, $C{{H}_{4}}$ . The carbon atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is $s{{p}^{3}}$ and the numbers of bonds are four then the structure of $C{{H}_{4}}$ is tetrahedral and it is as follows.
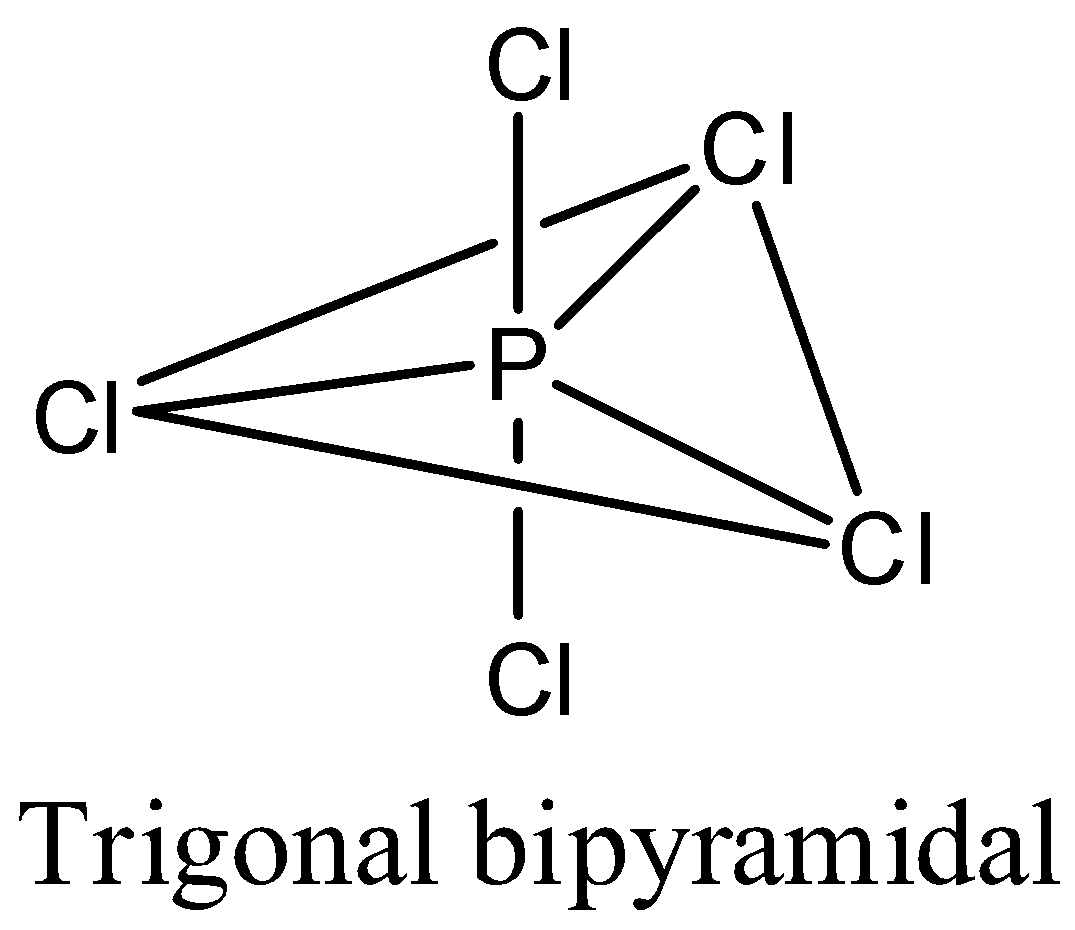
- Coming to the option C, $PC{{l}_{5}}$ . The phosphorus atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is $s{{p}^{3}}d$and the numbers of bonds are five then the structure of is trigonal bipyramidal and it is as follows.
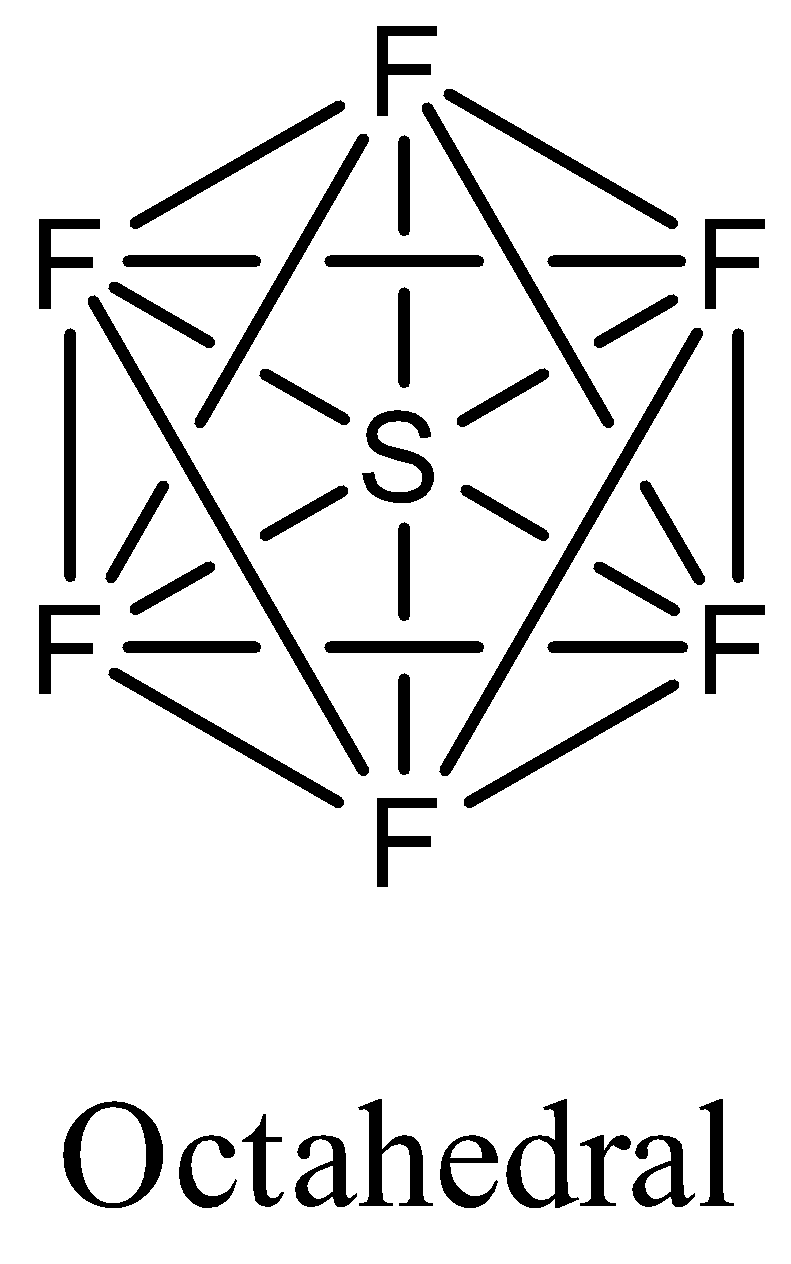
- Coming to the option D, $S{{F}_{6}}$. The sulfur atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is $s{{p}^{3}}{{d}^{2}}$ and the numbers of bonds are six then the structure of is octahedral and it is as follows.
- Therefore the molecule which has trigonal bipyramidal structure among the given options is $PC{{l}_{5}}$. So, the correct answer is “Option C”.
Note: If a lone pair of electrons are present on the central atom in the molecule then the shape of the molecule is going to change because of the repulsions between lone pair and bond pair electrons in the molecule.
Complete step by step answer:
- In the question, it is asked which molecules have the shape of trigonal bipyramidal among the given options.
- First, we should know the number of lone pairs on the central atom, the number of bonds, and the hybridization of the given molecules to find the shape of the given molecules.
- Coming to option A, $B{{F}_{3}}$. The boron atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is $s{{p}^{2}}$ and the number of bonds is three then the structure of $B{{F}_{3}}$ is trigonal planar and it is as follows.

- Coming to option B, $C{{H}_{4}}$ . The carbon atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is $s{{p}^{3}}$ and the numbers of bonds are four then the structure of $C{{H}_{4}}$ is tetrahedral and it is as follows.

- Coming to the option C, $PC{{l}_{5}}$ . The phosphorus atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is $s{{p}^{3}}d$and the numbers of bonds are five then the structure of is trigonal bipyramidal and it is as follows.

- Coming to the option D, $S{{F}_{6}}$. The sulfur atom does not contain any lone pair of electrons on the central atom, the hybridization of the molecule is $s{{p}^{3}}{{d}^{2}}$ and the numbers of bonds are six then the structure of is octahedral and it is as follows.

- Therefore the molecule which has trigonal bipyramidal structure among the given options is $PC{{l}_{5}}$. So, the correct answer is “Option C”.
Note: If a lone pair of electrons are present on the central atom in the molecule then the shape of the molecule is going to change because of the repulsions between lone pair and bond pair electrons in the molecule.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




