
Which of the following statements regarding the structure of $SOC{l_2}$ is not correct ?
( A ) The sulphur is $s{p^3}$ hybridised and it has a tetrahedral shape .
( B ) The sulphur is $s{p^3}$ hybridised and it has a trigonal pyramid shape .
( C ) The oxygen – sulphur bond is p$\pi $ – d$\pi $ bond .
( D ) It contains one lone pair of electrons in the $s{p^3}$ hybrid orbital of sulphur .
Answer
573.9k+ views
Hint: As three atoms are bonded to the sulphur atom, we can say that the sulphur atom is $s{p^3}$ hybridized.And by referring to the shapes of atoms of different hybridization, it’s a trigonal pyramid shape. The shape and geometry of the molecule are defined by minimum repulsion.
For 3 electron pairs, the shape is trigonal pyramidal.
Complete step by step solution:
First, we consider, option ( A )
The $SOC{l_2}$ molecule is $s{p^3}$ hybridized.
The formula for finding hybridization of molecules is
Hybridization $ = \dfrac{1}{2}\left( {V + H - C + A} \right)$
Here, V $ = $ VALENCE ELECTRONS IN CENTRAL METAL ATOM
H $ = $ Number of monovalent atom attached to the central metal atom
C $ = $ Cation charge
A $ = $ Anion charge
Substituting the values for $SOC{l_2}$,
$\Rightarrow $Hybridization $ = $ $\dfrac{1}{2}$( 6 $ + $2 $ + $0 $ + $0 )
$ = $$\dfrac{1}{2}$( 8 )
$ = $4
Hence, the hybridization of $SOC{l_2}$is $s{p^3}$
Now, the structure of $SOC{l_2}$ is a trigonal planar because of the electron densities of 2 chlorine atoms and 1 oxygen atom, as given in the image.
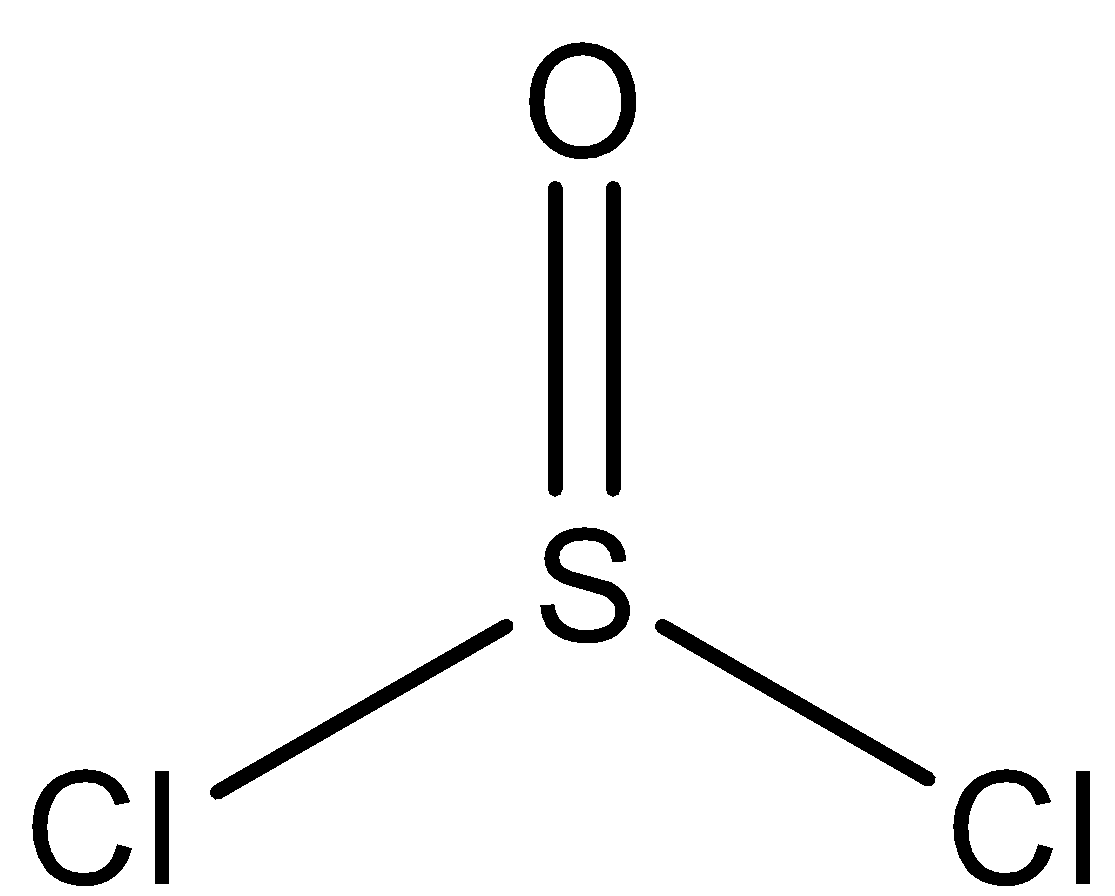
Hence, option ( A ) is incorrect
For option ( B ),
The statement is true, as proved above that the sulphur is $s{p^3}$ hybridized and it has a trigonal pyramid shape.
Hence, option ( B ) is correct
For option ( C ),
The lone pair of p orbital back bonds with that of d orbital of central atom sulphur.
Hence, option ( C ) is correct
For option ( D ),
The electronic configuration of the sulphur atom is
1${s^2}$ 2${s^2}$ 2${p^6}$ 3${s^2}$ 3${p^4}$
The 4 valence electrons of sulphur bond with 2 chlorine atoms and 1 oxygen atom leaving behind 2 valence electrons out of 6
Hence, the sulphur atom contains 1 lone pair of electrons
Hence, option ( D ) is correct
So, The correct option is ( A ) - The sulphur is $s{p^3}$ hybridized and it has a tetrahedral shape.
Note: The geometry of $SOC{l_2}$ is due to the lone pair effect. The VSEPR Theory ( Valence Shell Electron Pair Repulsion ) gives the geometry and shape of molecules.
According to the VSEPR Theory the bond angle between oxygen, sulphur and chlorine are $106^\circ $and between chlorine, sulphur, chlorine is $96^\circ $ respectively.
For 3 electron pairs, the shape is trigonal pyramidal.
Complete step by step solution:
First, we consider, option ( A )
The $SOC{l_2}$ molecule is $s{p^3}$ hybridized.
The formula for finding hybridization of molecules is
Hybridization $ = \dfrac{1}{2}\left( {V + H - C + A} \right)$
Here, V $ = $ VALENCE ELECTRONS IN CENTRAL METAL ATOM
H $ = $ Number of monovalent atom attached to the central metal atom
C $ = $ Cation charge
A $ = $ Anion charge
Substituting the values for $SOC{l_2}$,
$\Rightarrow $Hybridization $ = $ $\dfrac{1}{2}$( 6 $ + $2 $ + $0 $ + $0 )
$ = $$\dfrac{1}{2}$( 8 )
$ = $4
Hence, the hybridization of $SOC{l_2}$is $s{p^3}$
Now, the structure of $SOC{l_2}$ is a trigonal planar because of the electron densities of 2 chlorine atoms and 1 oxygen atom, as given in the image.

Hence, option ( A ) is incorrect
For option ( B ),
The statement is true, as proved above that the sulphur is $s{p^3}$ hybridized and it has a trigonal pyramid shape.
Hence, option ( B ) is correct
For option ( C ),
The lone pair of p orbital back bonds with that of d orbital of central atom sulphur.
Hence, option ( C ) is correct
For option ( D ),
The electronic configuration of the sulphur atom is
1${s^2}$ 2${s^2}$ 2${p^6}$ 3${s^2}$ 3${p^4}$
The 4 valence electrons of sulphur bond with 2 chlorine atoms and 1 oxygen atom leaving behind 2 valence electrons out of 6
Hence, the sulphur atom contains 1 lone pair of electrons
Hence, option ( D ) is correct
So, The correct option is ( A ) - The sulphur is $s{p^3}$ hybridized and it has a tetrahedral shape.
Note: The geometry of $SOC{l_2}$ is due to the lone pair effect. The VSEPR Theory ( Valence Shell Electron Pair Repulsion ) gives the geometry and shape of molecules.
According to the VSEPR Theory the bond angle between oxygen, sulphur and chlorine are $106^\circ $and between chlorine, sulphur, chlorine is $96^\circ $ respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




