
Which of the following shows bonds in silicone?
(A) Si – C – Si – C – Si
(B) Si – Si – Si – Si
(C) Si – O – Si – O – Si
(D) Si – C – Si – O – Si
Answer
579.6k+ views
Hint: Silicones are the organo-silicon polymers which are exclusively made of carbon, silicon and oxygen atoms. The repeating unit of all the silicones is $ - {R_2}SiO - $ where R is an alkyl group.
Complete answer:
We will get some information about silicone.
- Silicones are a group of organo-silicon polymers as they are exclusively made up of carbon, silicon and oxygen atoms.
- All the silicons have $ - {R_2}SiO - $ as a repeating unit.
- Silicones are hydrophobic in nature because of the presence of alkyl groups on silicon atoms. Generally, they have high dielectric strength and high thermal stability. They are resistant to oxidation.
- Due to these properties, they are widely used in sealants, insulators, greases and fabrics which are used for waterproofing. They are biocompatible also. So, they are also used in surgical plants.
- The structure of silicones can be given by
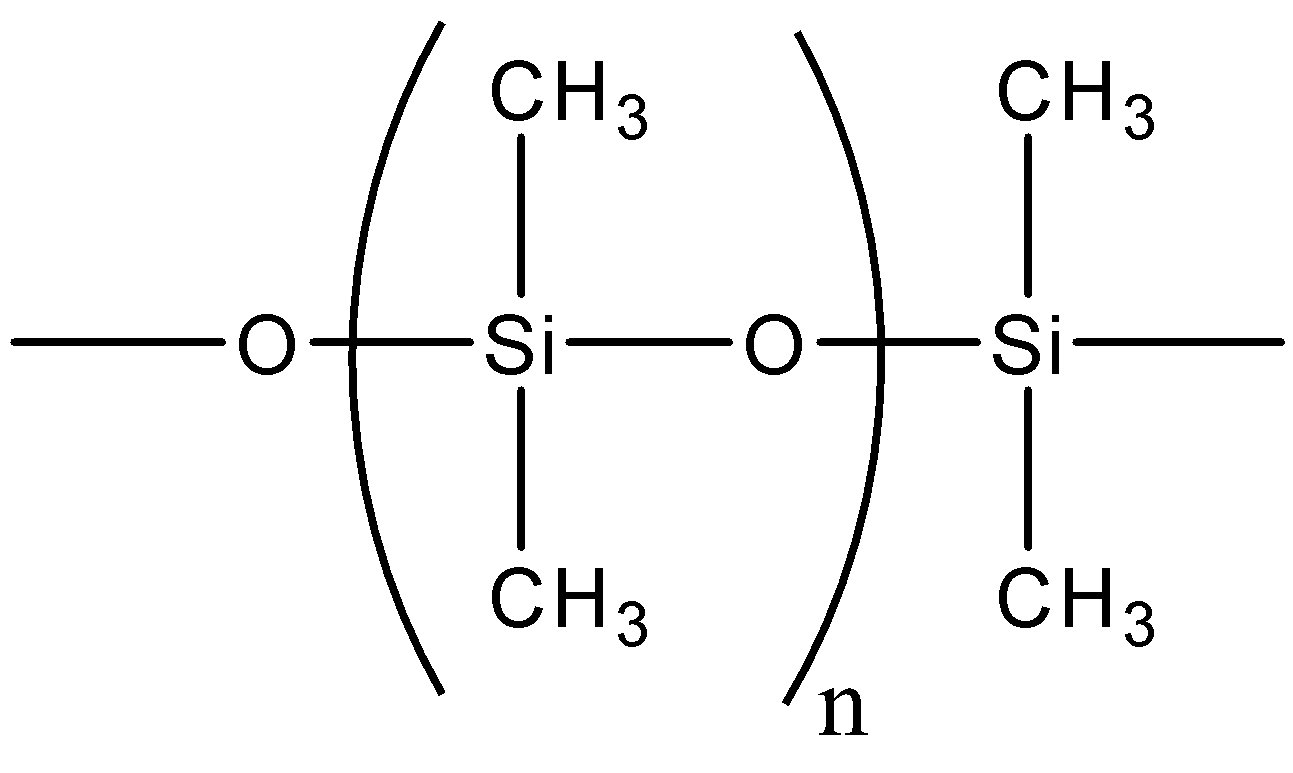
- Silicon atoms are tetravalent as silicon is a carbon family element. Oxygen is a divalent element. Here, we can see that each silicon atom is bonded to two oxygen atoms and two carbon atoms. So, there are two methyl groups bonded to each silicon atom in silicones.
- So, from the above structure. We can say that the bonding between silicon and oxygen atoms is like Si – O – Si – O – Si .
Thus, the correct answer is (C).
Note:
Do not get confused with silica which has a molecular formula of $Si{O_2}$ and it also involves Si – O – Si – O – Si linkage. Note that silicates are the compounds in which silicon exists in anion $Si{O_4}^{4 - }$.
Complete answer:
We will get some information about silicone.
- Silicones are a group of organo-silicon polymers as they are exclusively made up of carbon, silicon and oxygen atoms.
- All the silicons have $ - {R_2}SiO - $ as a repeating unit.
- Silicones are hydrophobic in nature because of the presence of alkyl groups on silicon atoms. Generally, they have high dielectric strength and high thermal stability. They are resistant to oxidation.
- Due to these properties, they are widely used in sealants, insulators, greases and fabrics which are used for waterproofing. They are biocompatible also. So, they are also used in surgical plants.
- The structure of silicones can be given by

- Silicon atoms are tetravalent as silicon is a carbon family element. Oxygen is a divalent element. Here, we can see that each silicon atom is bonded to two oxygen atoms and two carbon atoms. So, there are two methyl groups bonded to each silicon atom in silicones.
- So, from the above structure. We can say that the bonding between silicon and oxygen atoms is like Si – O – Si – O – Si .
Thus, the correct answer is (C).
Note:
Do not get confused with silica which has a molecular formula of $Si{O_2}$ and it also involves Si – O – Si – O – Si linkage. Note that silicates are the compounds in which silicon exists in anion $Si{O_4}^{4 - }$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




