
Which of the following resonating structures will contribute minimum to resonance hybrid?
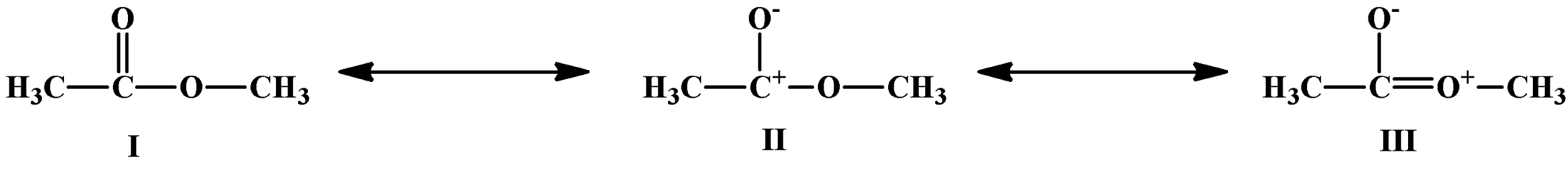
(A) I
(B) II
(C) III
(D) All the structures contribute equally
Answer
592.2k+ views
Hint: All the canonical structures equivalently share the overall energy of the molecule. They contribute equally to the resonance hybrid.
Complete step-by-step answer:
Let us talk about resonance first. Resonance can be defined as the permanent delocalisation of electrons within a molecule between conjugated pi-bonds. We define this dynamic phenomenon as a permanent effect because it is not limited to any structure or condition. A molecule which can resonate continuously does so, until it does not.
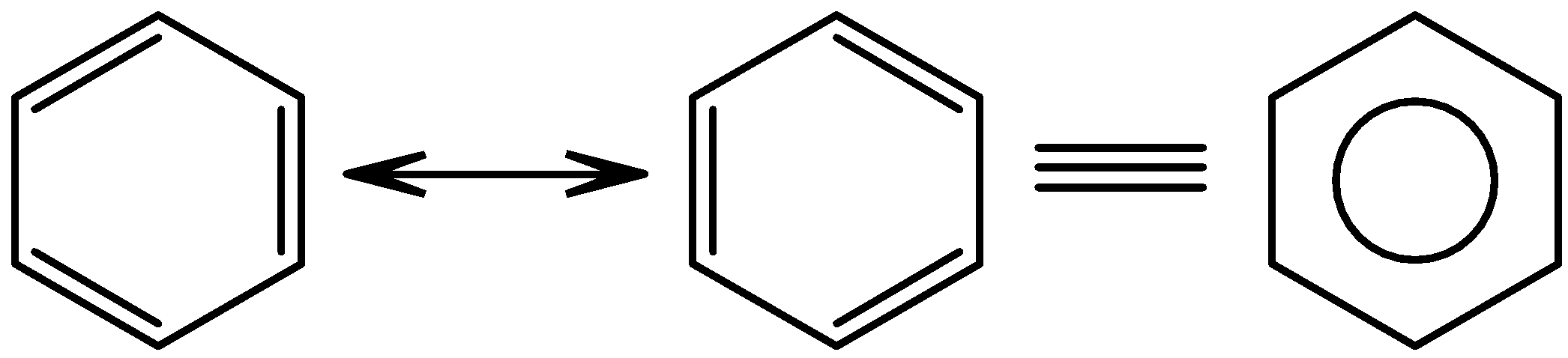
Not all molecules which have conjugated pi-bonds resonate. Only a certain group of compounds which are known as aromatic compounds do so. We can identify these compounds with a special rule known as Huckel’s rule. It states that molecules which contain $\left( 4n+2 \right)\pi $electrons, where n is any natural number, are regarded as aromatic compounds. If we put n is equal to 1 then we get the result as 6 pi-electrons. Benzene, which is a very common molecule in organic chemistry, has 6 pi-electrons and therefore is an aromatic compound.
Now, these structures which resonate go through intermediate states which are relatively unstable or short lived. These intermediate states are also known as canonical structures. The number of canonical structures is directly proportional to the stability of the overall compound. The molecule benzene in the above case has two such structures. But the actual structure of benzene is somewhere in between, in other words, the two canonical structures contribute equally to the actual structure of benzene. The overall structure of any resonating molecule is known as the resonance hybrid and is derived from its canonical structures. The resonance of benzene is as shown below-
Therefore, in the above question, the answer is option (D) All the structures contribute equally. The resonance hybrid of the given molecule is as shown below-
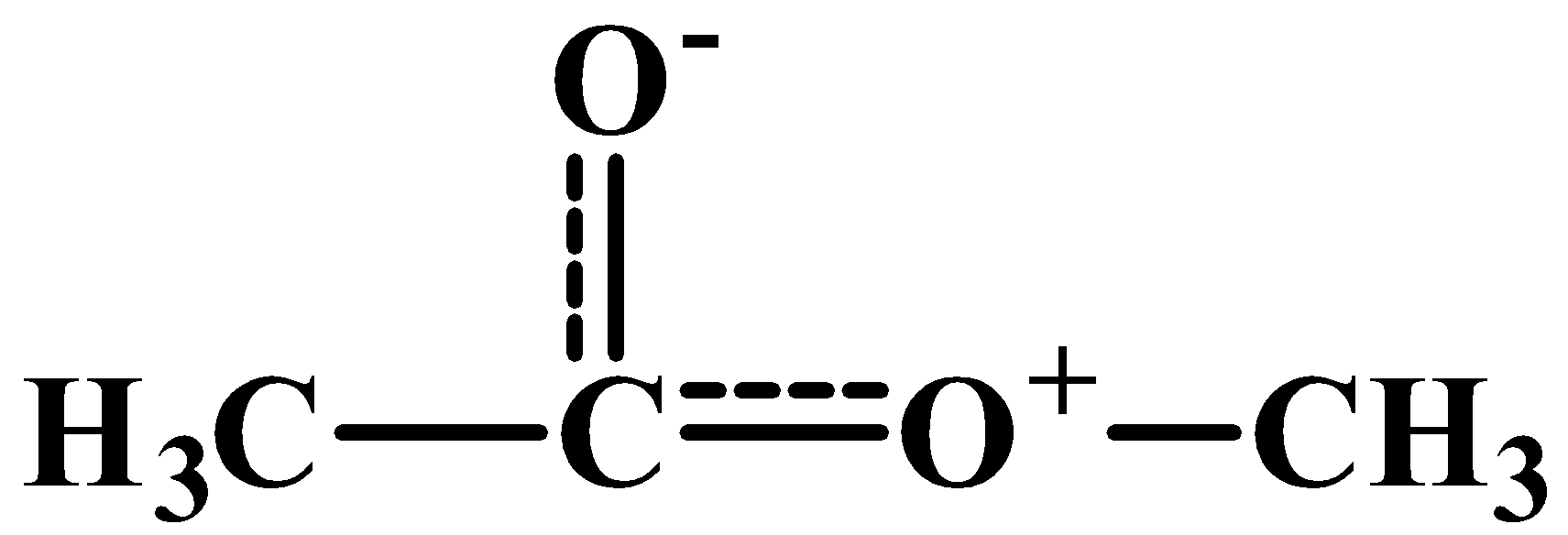
As you can see, both the bonds have partial double bond character.
Note: There is a difference between conjugation of double bonds and resonance. There are many compounds which show conjugation but do not resonate. Resonance is regarded as a permanent effect and has the highest stabilizing potential among all the other effects.
Complete step-by-step answer:
Let us talk about resonance first. Resonance can be defined as the permanent delocalisation of electrons within a molecule between conjugated pi-bonds. We define this dynamic phenomenon as a permanent effect because it is not limited to any structure or condition. A molecule which can resonate continuously does so, until it does not.
Not all molecules which have conjugated pi-bonds resonate. Only a certain group of compounds which are known as aromatic compounds do so. We can identify these compounds with a special rule known as Huckel’s rule. It states that molecules which contain $\left( 4n+2 \right)\pi $electrons, where n is any natural number, are regarded as aromatic compounds. If we put n is equal to 1 then we get the result as 6 pi-electrons. Benzene, which is a very common molecule in organic chemistry, has 6 pi-electrons and therefore is an aromatic compound.
Now, these structures which resonate go through intermediate states which are relatively unstable or short lived. These intermediate states are also known as canonical structures. The number of canonical structures is directly proportional to the stability of the overall compound. The molecule benzene in the above case has two such structures. But the actual structure of benzene is somewhere in between, in other words, the two canonical structures contribute equally to the actual structure of benzene. The overall structure of any resonating molecule is known as the resonance hybrid and is derived from its canonical structures. The resonance of benzene is as shown below-

Therefore, in the above question, the answer is option (D) All the structures contribute equally. The resonance hybrid of the given molecule is as shown below-

As you can see, both the bonds have partial double bond character.
Note: There is a difference between conjugation of double bonds and resonance. There are many compounds which show conjugation but do not resonate. Resonance is regarded as a permanent effect and has the highest stabilizing potential among all the other effects.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




