
Which of the following reactions is a part of Serpeck’s process?
A.$A{l_2}{O_3} + 2NaOH \to 2NaAl{O_2} + {H_2}O$
B.$F{e_2}{O_3} + 2Al \to 2Fe + A{l_2}{O_3}$
C.$AlN + 3{H_2}O \to Al{(OH)_3} + N{H_3}$
D.$A{l_2}{O_3}.2{H_2}O + 2N{a_2}C{O_3} \to 2NaAl{O_2} + C{O_2} + 2{H_2}O$
Answer
571.8k+ views
Hint: We know that Serpeck’s process is used for purification of Bauxite. Bauxite contains impurities of silica in excess amount, which needs to be removed. The bauxite powder is mixed with carbon and heated under high temperature (above $1800^\circ C$) and a current of nitrogen is passed. Through this process aluminium nitride is obtained along with water and carbon monoxide.
For the given question, let’s understand how the process works and then identify the reaction matching the process.
Complete step by step solution:
First let us understand the reactions involved in Serpeck’s process.
Bauxite powder is strongly heated in the presence of coke and nitrogen gas to produce aluminium nitride along with carbon monoxide and water. As water is present, the aluminium nitride undergoes hydrolysis to form Aluminium hydroxide. When heated strongly this aluminium produces alumina.
The complete reaction can be written as:
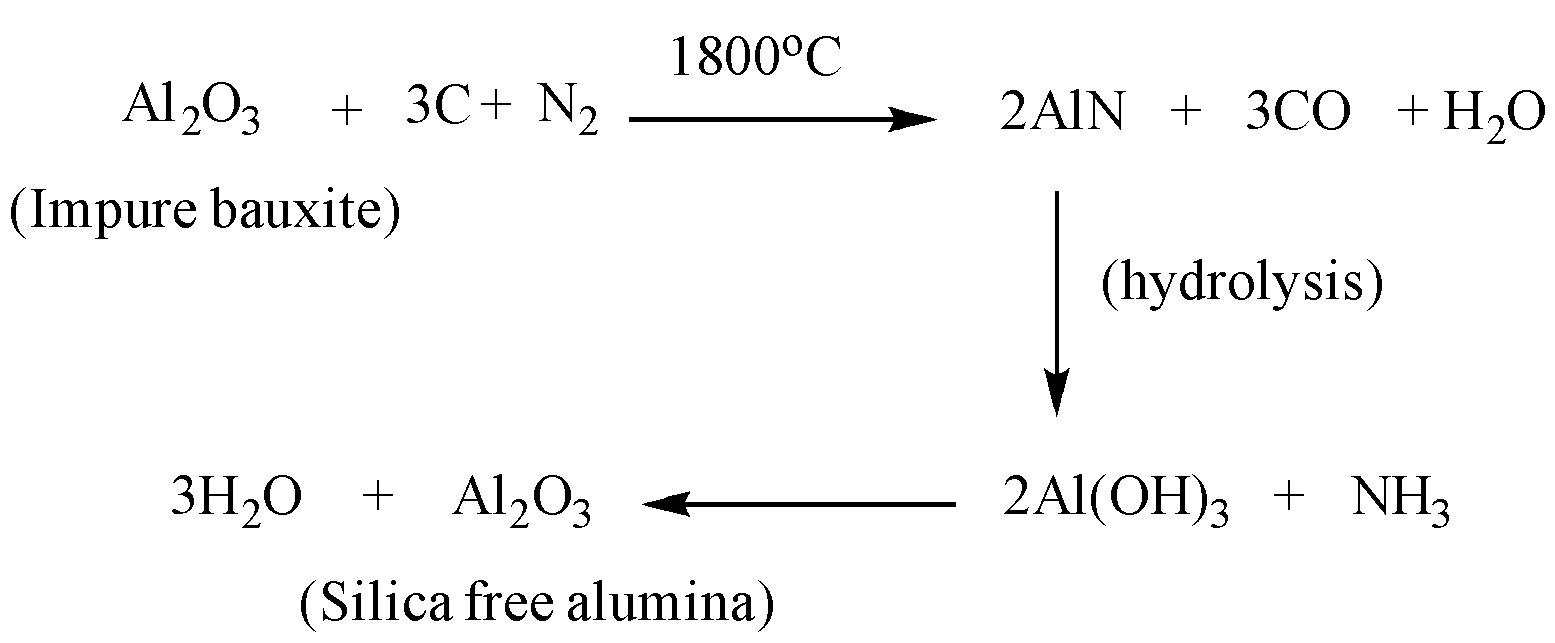
Meanwhile the silica present in the bauxite, reacts with carbon to form silicon and carbon dioxide. The reaction can be represented as:
$Si{O_2} + C \to Si + 2C{O_2}$
Hence, through this process pure alumina is obtained.
Among the reactions given in the question, there is one reaction which is a part of this process.
The reaction is – $AlN + 3{H_2}O \to Al{(OH)_3} + N{H_3}$
This process is the hydrolysis of aluminium nitride to form aluminium hydroxide and ammonia.
Whereas any other reaction doesn’t show any resemblance to the reactions involved in Serpeck’s process.
Hence, the correct answer to the question is option C.
Note:As we know that the bauxite is found in nature, which contains alumina along with other impurities especially silica in big amounts. To remove this silica, there are various methods such as Bayer’s process and Hall-Heroult process. The Bayer’s process is the primary process to extract alumina. In the Hall-Heroult process alumina undergoes a smelting process.
For the given question, let’s understand how the process works and then identify the reaction matching the process.
Complete step by step solution:
First let us understand the reactions involved in Serpeck’s process.
Bauxite powder is strongly heated in the presence of coke and nitrogen gas to produce aluminium nitride along with carbon monoxide and water. As water is present, the aluminium nitride undergoes hydrolysis to form Aluminium hydroxide. When heated strongly this aluminium produces alumina.
The complete reaction can be written as:

Meanwhile the silica present in the bauxite, reacts with carbon to form silicon and carbon dioxide. The reaction can be represented as:
$Si{O_2} + C \to Si + 2C{O_2}$
Hence, through this process pure alumina is obtained.
Among the reactions given in the question, there is one reaction which is a part of this process.
The reaction is – $AlN + 3{H_2}O \to Al{(OH)_3} + N{H_3}$
This process is the hydrolysis of aluminium nitride to form aluminium hydroxide and ammonia.
Whereas any other reaction doesn’t show any resemblance to the reactions involved in Serpeck’s process.
Hence, the correct answer to the question is option C.
Note:As we know that the bauxite is found in nature, which contains alumina along with other impurities especially silica in big amounts. To remove this silica, there are various methods such as Bayer’s process and Hall-Heroult process. The Bayer’s process is the primary process to extract alumina. In the Hall-Heroult process alumina undergoes a smelting process.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




