
Which of the following molecules has no dative bond?
A.$CO$
B.$C{O_3}^{2 - }$
C.$S{O_4}^{2 - }$
D.All of these
Answer
517.5k+ views
Hint: We know that a covalent bond is formed when two molecules share electrons. The electron pair is pulled into both nuclear cores, holding them collected to result in a bond. In general covalent bond, every atom contributes an electron for the formation of bond.
Complete answer:
We can define a dative bond as a covalent bond which is formed between two atoms. Here, one of the atoms gives both electrons which form the bond. Coordinate bond (or) dipolar bond is another name for a dative bond. We can say that a dative bond is a two center, two electron covalent bond where both electrons come from similar atoms. When ions of metal are bound to ligands, dative bonds generally occur.
Another condition for the formation of dative bonds is that one lone pair of electrons should be found in one of atoms that is covalently bonded to another atom.
The molecule which does not have dative bond is $C{O_3}^{2 - }$. We can explain the reason for this by their arrangement of atoms. We can draw the structure of $C{O_3}^{2 - }$ as,
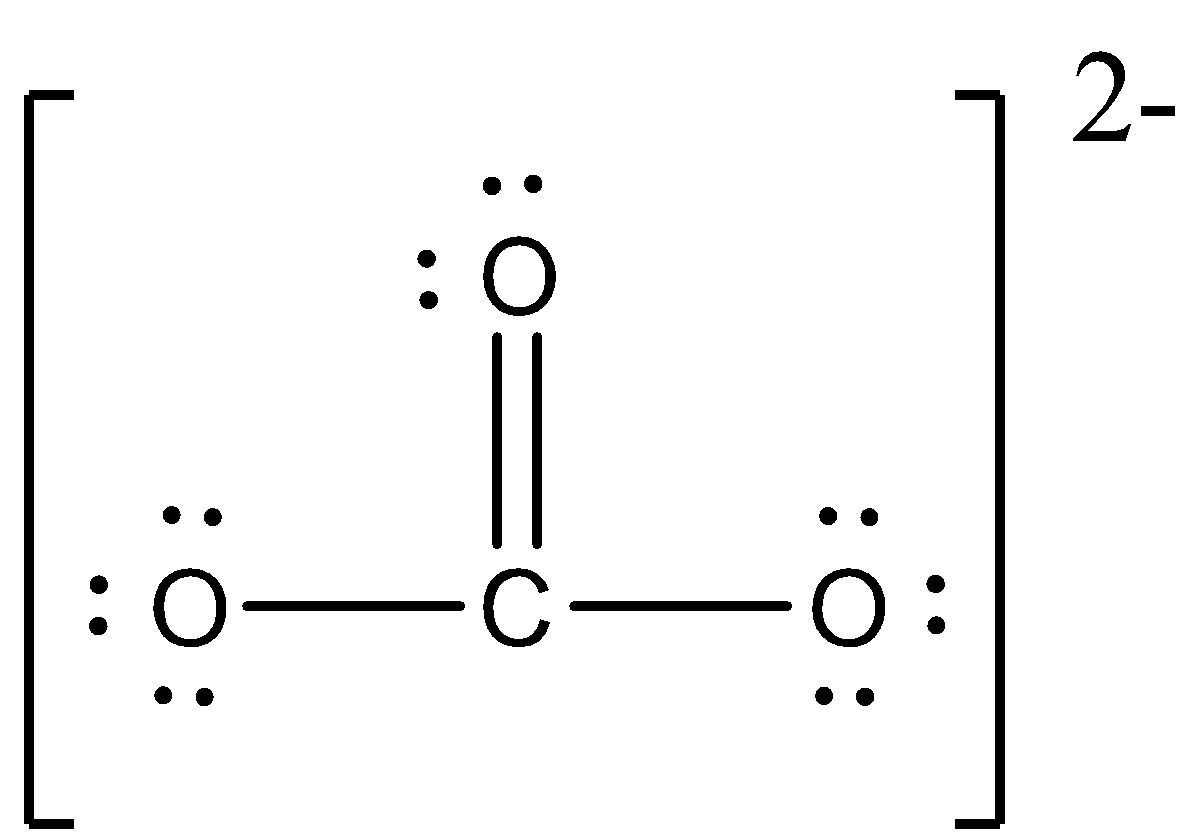
We can see that an atom of carbon is doubly bonded to an atom of oxygen which is neutral, and carbon atom is singly bonded to oxygen that has negative charge. These single bonded oxygen requires one more electron to attain their octet and on other side, in the molecule of $C = O$, the atom of electron contains two electrons to be shared, so each of the oxygen atoms that are negatively charge share their electrons with atom of carbon. So, this molecule does not contain dative bonds.
Option (2) is correct.
Note:
We have to know that in diagrammatic representation, we can indicate a dative bond by drawing an arrow indicating from the atom which gives the lone pair of electrons towards that atom which gains the pair. The arrow displaces the line that represents chemical bonds. Generally, we can see dative bonds in those reactions that involve atoms of hydrogen.
Complete answer:
We can define a dative bond as a covalent bond which is formed between two atoms. Here, one of the atoms gives both electrons which form the bond. Coordinate bond (or) dipolar bond is another name for a dative bond. We can say that a dative bond is a two center, two electron covalent bond where both electrons come from similar atoms. When ions of metal are bound to ligands, dative bonds generally occur.
Another condition for the formation of dative bonds is that one lone pair of electrons should be found in one of atoms that is covalently bonded to another atom.
The molecule which does not have dative bond is $C{O_3}^{2 - }$. We can explain the reason for this by their arrangement of atoms. We can draw the structure of $C{O_3}^{2 - }$ as,

We can see that an atom of carbon is doubly bonded to an atom of oxygen which is neutral, and carbon atom is singly bonded to oxygen that has negative charge. These single bonded oxygen requires one more electron to attain their octet and on other side, in the molecule of $C = O$, the atom of electron contains two electrons to be shared, so each of the oxygen atoms that are negatively charge share their electrons with atom of carbon. So, this molecule does not contain dative bonds.
Option (2) is correct.
Note:
We have to know that in diagrammatic representation, we can indicate a dative bond by drawing an arrow indicating from the atom which gives the lone pair of electrons towards that atom which gains the pair. The arrow displaces the line that represents chemical bonds. Generally, we can see dative bonds in those reactions that involve atoms of hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




