
Which of the following is the weakest Bronsted base?
(A)-
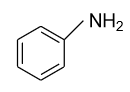
(B)-
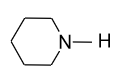
(C)-
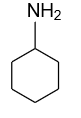
(D)- \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}\]



Answer
560.4k+ views
Hint: Bronsted – Lowry theory is also known by the name of acid-base theory and according to this theory, those species which accept protons are the bases and those which donate protons are acid.
Complete step by step answer:
It is clear that Bronsted bases are the species that accept protons towards them from the outside. So among them, those bases which easily or eagerly accept protons will come under the category of strong bases, and those which don't show a keen interest in accepting protons will come under the category of weak bases.
Order of basicity of amines is shown as follow:
${\text{N}}{{\text{H}}_{\text{3}}}$ < Primary or tertiary amine < Secondary amine
-In option (A) primary amine i.e. aniline is given and lone pair of an electron which presents in the nitrogen atom (${\text{N}}$) is engaged in the delocalization process with the benzene ring and will show very less interest towards accepting of the proton. So, aniline comes under the category of a weak base.
-In the option (C) and (D) also primary amines are given but their basicity is usually more than aniline because attached alkyl group shows +I effect and nitrogen atom will show –I effect, due to which in an aqueous form they will accept proton easily and shows strong basicity.
-In option (B) secondary amine is given and it shows the highest basicity due to the presence of two +I effects showing groups to the nitrogen atom of amine.
So, from the above discussion, it is clear that aniline is the weakest base. So, the correct answer is “Option A”.
Note: Here some of you may think that cyclohexylamine is showing strong basicity whether this was also attached with the ring. So the reason is that in this ring a double bond is not present to develop conjugation, that’s why it accepts protons easily.
Complete step by step answer:
It is clear that Bronsted bases are the species that accept protons towards them from the outside. So among them, those bases which easily or eagerly accept protons will come under the category of strong bases, and those which don't show a keen interest in accepting protons will come under the category of weak bases.
Order of basicity of amines is shown as follow:
${\text{N}}{{\text{H}}_{\text{3}}}$ < Primary or tertiary amine < Secondary amine
-In option (A) primary amine i.e. aniline is given and lone pair of an electron which presents in the nitrogen atom (${\text{N}}$) is engaged in the delocalization process with the benzene ring and will show very less interest towards accepting of the proton. So, aniline comes under the category of a weak base.
-In the option (C) and (D) also primary amines are given but their basicity is usually more than aniline because attached alkyl group shows +I effect and nitrogen atom will show –I effect, due to which in an aqueous form they will accept proton easily and shows strong basicity.
-In option (B) secondary amine is given and it shows the highest basicity due to the presence of two +I effects showing groups to the nitrogen atom of amine.
So, from the above discussion, it is clear that aniline is the weakest base. So, the correct answer is “Option A”.
Note: Here some of you may think that cyclohexylamine is showing strong basicity whether this was also attached with the ring. So the reason is that in this ring a double bond is not present to develop conjugation, that’s why it accepts protons easily.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




