
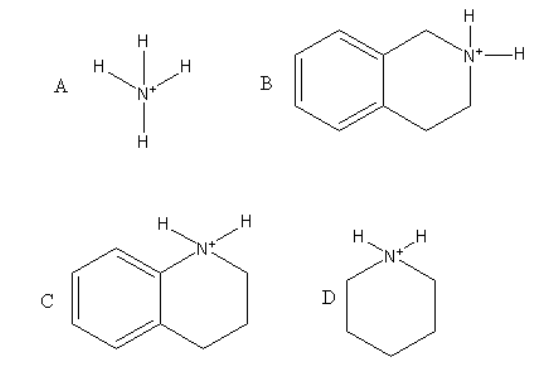
Which of the following is the strongest acid?

Answer
573.9k+ views
Hint: The basicity is inversely proportional to the acidity. In the case of the base, the acidity is determined by checking the basic strength of the bases. Least the basicity high will be the acidity.
Complete Step by step answer: The given compounds are protonated amines. Amines are basic because amines have lone pair the nitrogen atom which can be donated.
The amine which is the weakest base will be the strongest acid because that amine cannot donate proton easily, so that amine will accept proton hence will be acidic.
So, we will remove a proton form all the given amines and check their basicity as follows:

Among all four piperidine is the strongest base because it has lone pair at nitrogen which has ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization and all other carbons of the ring are also ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized so, all are electron donating hence it is most basic.
Piperidine is a stronger base than ammonia and $1,2,3,4 - $tetrahydroisoquinoline because in ammonia inversion is found which decrease its basicity and in $1,2,3,4 - $ tetrahydroisoquinoline the electron-withdrawing effect of the attached benzene ring decreases the basicity.
$1,2,3,4 - $ Tetrahydroquinoline is the least basic because the lone pair present at nitrogen are delocalised in the attached benzene ring. So, the availability of the lone pair decreases which decreases the basic strength of the $1,2,3,4 - $tetrahydroquinoline.
So, $1,2,3,4 - $ tetrahydroquinoline will be the strongest acid.
Therefore, option (C) is correct.
Note: In the case of acids, the acidity is determined by checking the stability of the anion formed by the removal of the proton. More stable the anion formed by removal of the proton will be more acidic. The factors that stabilize the anion formed by the removal of protons increase the acidity. Resonance decreases the basicity. Weaker the base stronger the acid or vice versa.
Complete Step by step answer: The given compounds are protonated amines. Amines are basic because amines have lone pair the nitrogen atom which can be donated.
The amine which is the weakest base will be the strongest acid because that amine cannot donate proton easily, so that amine will accept proton hence will be acidic.
So, we will remove a proton form all the given amines and check their basicity as follows:

Among all four piperidine is the strongest base because it has lone pair at nitrogen which has ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization and all other carbons of the ring are also ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized so, all are electron donating hence it is most basic.
Piperidine is a stronger base than ammonia and $1,2,3,4 - $tetrahydroisoquinoline because in ammonia inversion is found which decrease its basicity and in $1,2,3,4 - $ tetrahydroisoquinoline the electron-withdrawing effect of the attached benzene ring decreases the basicity.
$1,2,3,4 - $ Tetrahydroquinoline is the least basic because the lone pair present at nitrogen are delocalised in the attached benzene ring. So, the availability of the lone pair decreases which decreases the basic strength of the $1,2,3,4 - $tetrahydroquinoline.
So, $1,2,3,4 - $ tetrahydroquinoline will be the strongest acid.
Therefore, option (C) is correct.
Note: In the case of acids, the acidity is determined by checking the stability of the anion formed by the removal of the proton. More stable the anion formed by removal of the proton will be more acidic. The factors that stabilize the anion formed by the removal of protons increase the acidity. Resonance decreases the basicity. Weaker the base stronger the acid or vice versa.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




