
Which of the following is not correctly matched?
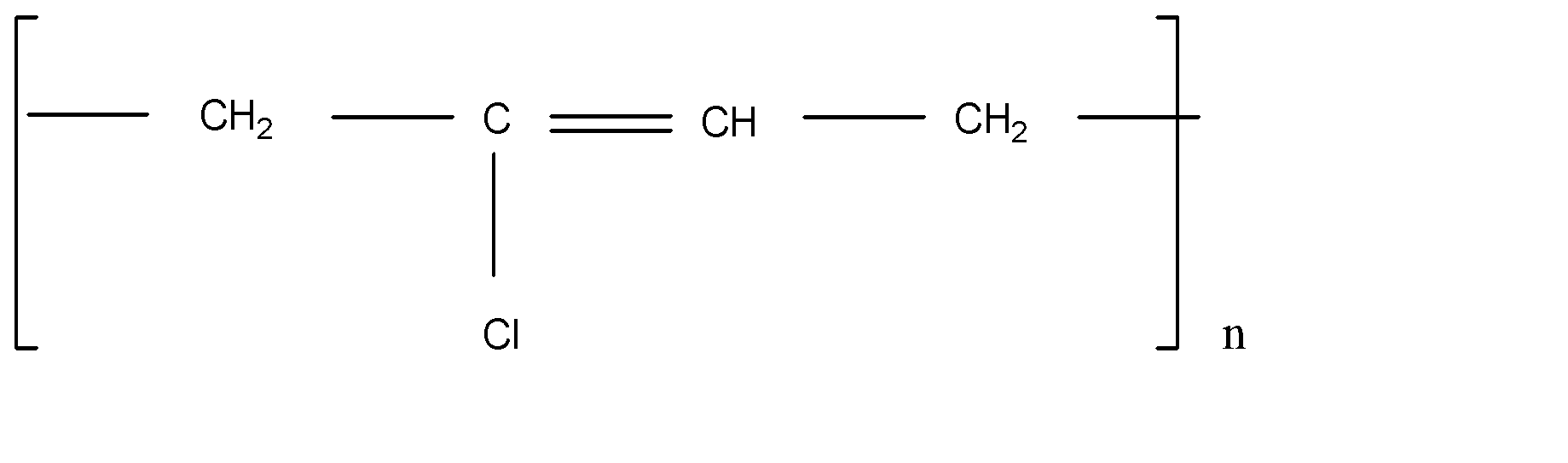
A.Neoprene
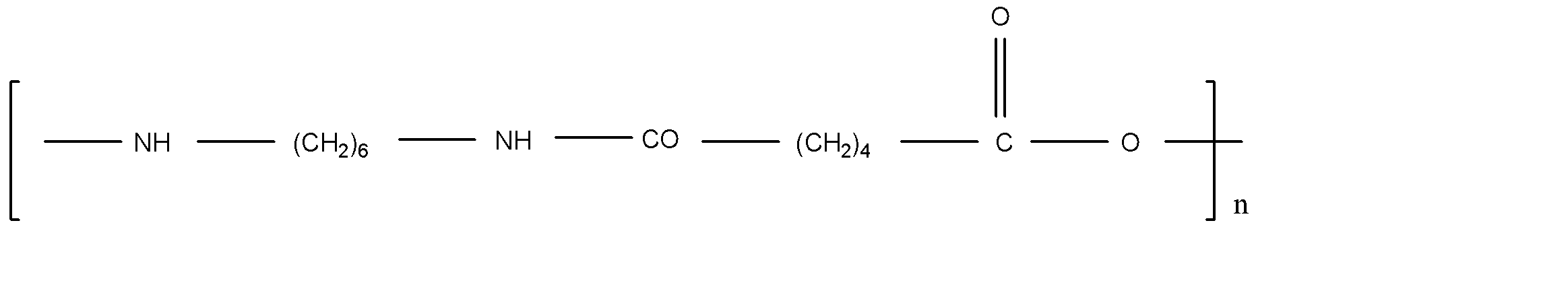
B.Nylon 6,6
 C.Terylene
C.Terylene
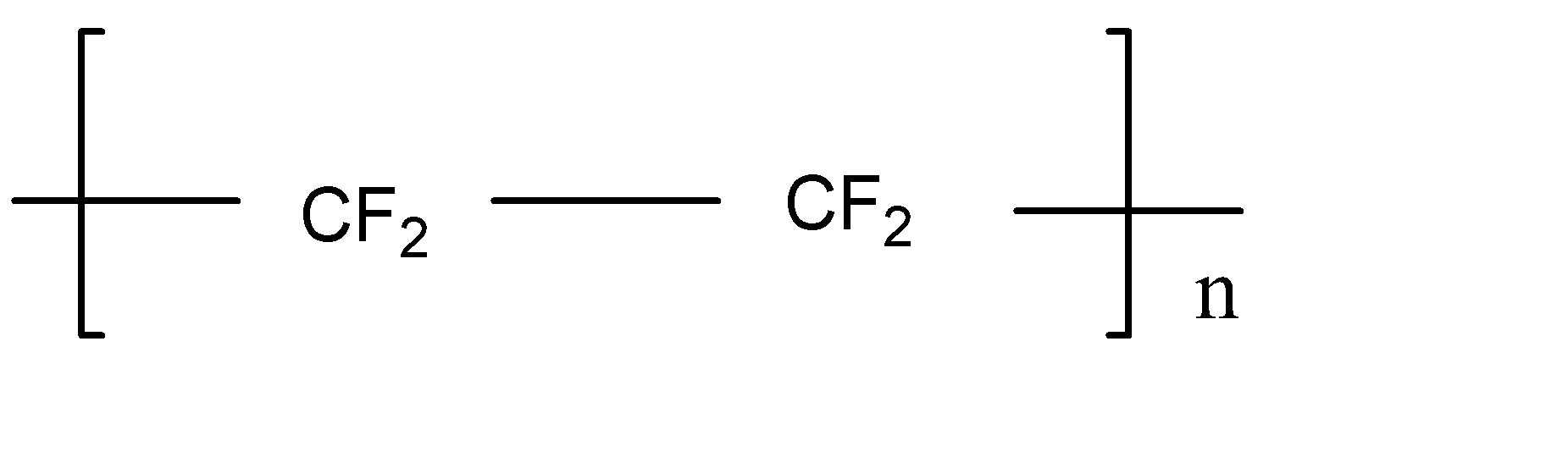
D.Teflon



Answer
581.1k+ views
Hint: Polymers are long chain compounds, which are repeating chains of molecules. Polymers are a large number of molecules which are made by chemical bonding. Here we see the examples of polymers and find the option which is not correctly matched by repeat molecules and form polymers. Neoprene, Nylon 6,6 and Teflon are given in the long chain. In these polymers, molecules are repeating and form long chain molecules.
Complete step by step answer:
Here we see the example of polymers, we find the polymer which is not correctly matched. The examples of polymers are given by structure in this option. Here Terylene is not described by the structure.
Nylon 6,6 is the polyamide polymer. The monomer in nylon 6,6 is polyamide. Neoprene is the polychloroprene and it exhibits good chemical stability and maintains flexibility. Ethylene glycol and terephthalic acid react and form Terylene by polymerization. Teflon is a polytetrafluoroethylene polymer.
Hence, option (C) is the correct answer.
Additional information:
Polymer is a useful chemical made of many repeating units. It is made up of many of the materials in living organisms, including proteins, cellulose and nucleic acids. Many important polymers have nitrogen or oxygen atoms. The nylon and polyester in jackets or sneakers. Polymers are large molecules made by bonding.
Note: Here Terylene is the polymer between two molecules, but other polymers are the long chain of single molecules. It forms by the single molecule by repeat. Nylon 6,6, Neoprene and Teflon is formed by the single molecule and Terylene is the formation of two different molecules. That’s why Terylene is different from others.
Complete step by step answer:
Here we see the example of polymers, we find the polymer which is not correctly matched. The examples of polymers are given by structure in this option. Here Terylene is not described by the structure.
Nylon 6,6 is the polyamide polymer. The monomer in nylon 6,6 is polyamide. Neoprene is the polychloroprene and it exhibits good chemical stability and maintains flexibility. Ethylene glycol and terephthalic acid react and form Terylene by polymerization. Teflon is a polytetrafluoroethylene polymer.
Hence, option (C) is the correct answer.
Additional information:
Polymer is a useful chemical made of many repeating units. It is made up of many of the materials in living organisms, including proteins, cellulose and nucleic acids. Many important polymers have nitrogen or oxygen atoms. The nylon and polyester in jackets or sneakers. Polymers are large molecules made by bonding.
Note: Here Terylene is the polymer between two molecules, but other polymers are the long chain of single molecules. It forms by the single molecule by repeat. Nylon 6,6, Neoprene and Teflon is formed by the single molecule and Terylene is the formation of two different molecules. That’s why Terylene is different from others.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




