
Which of the following is not an example of an additional polymer?
(A)Polythene
(B)Polystyrene
(C)Neoprene
(D)Terylene
Answer
581.7k+ views
Hint: Polyesters are formed by condensation polymerization. Additional polymerization and condensation are two different types of polymerization reactions. In the addition polymerization the monomer units are added into a chain without the loss of any molecules.
Complete Step by step solution:
Polymerization is a process in which many small or simple units of one or more compounds combine with each other to give new larger chains of compounds called polymers. Polymerization is of different types such as addition polymerization, condensation polymerization.
-Additional polymerization is the process in which the monomer units are joined to each other to form continuous chains without giving any other product. This is also called chain growth polymerization. Generally the additional polymerization is done to the monomers having double bonds, where the bonds break to form new bonds with the incoming monomer.
-The condensation polymerization also results in co-products of small molecules such as water (${{\text{H}}_{\text{2}}}{\text{O}}$) molecules, ammonia (${\text{N}}{{\text{H}}_{\text{3}}}$) etc., along with the polymers.
In the above given options, polythene is a synthetic polymer, a plastic made up of ethylene monomer units. It is a homo polymer i.e. it contains a single type of monomer and is made by addition polymerization. Polystyrene is also an additional polymer with styrene units added by anionic polymerization. Neoprene is a synthetic rubber made from chloroprene units by free radical mechanism.
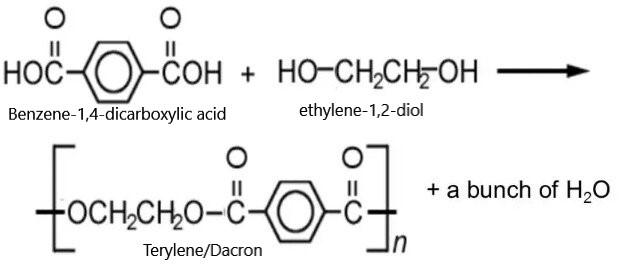
Coming to Terylene, it is a polyester commonly known as PET (poly ethyl terephthalate). Polyesters are made by the reaction between an acid monomer and an alcohol containing monomer. The reaction proceeds by formation of ester linkages resulting in the release of water molecules. This type of polymerization is known as condensation polymerization.
So the answer for the given question is option (D) Terylene.
NOTE: Polyesters can be mentioned as an example of condensation polymerization. Terylene is a polyester formed by the reaction between ethane-1,2-diol and benezene-1,4-dicarboxylic acid. Hydrogen ion from the alcohol and hydroxyl ion from the acid react to form water molecules along with Terylene polymer.
Complete Step by step solution:
Polymerization is a process in which many small or simple units of one or more compounds combine with each other to give new larger chains of compounds called polymers. Polymerization is of different types such as addition polymerization, condensation polymerization.
-Additional polymerization is the process in which the monomer units are joined to each other to form continuous chains without giving any other product. This is also called chain growth polymerization. Generally the additional polymerization is done to the monomers having double bonds, where the bonds break to form new bonds with the incoming monomer.
-The condensation polymerization also results in co-products of small molecules such as water (${{\text{H}}_{\text{2}}}{\text{O}}$) molecules, ammonia (${\text{N}}{{\text{H}}_{\text{3}}}$) etc., along with the polymers.
In the above given options, polythene is a synthetic polymer, a plastic made up of ethylene monomer units. It is a homo polymer i.e. it contains a single type of monomer and is made by addition polymerization. Polystyrene is also an additional polymer with styrene units added by anionic polymerization. Neoprene is a synthetic rubber made from chloroprene units by free radical mechanism.

Coming to Terylene, it is a polyester commonly known as PET (poly ethyl terephthalate). Polyesters are made by the reaction between an acid monomer and an alcohol containing monomer. The reaction proceeds by formation of ester linkages resulting in the release of water molecules. This type of polymerization is known as condensation polymerization.
So the answer for the given question is option (D) Terylene.
NOTE: Polyesters can be mentioned as an example of condensation polymerization. Terylene is a polyester formed by the reaction between ethane-1,2-diol and benezene-1,4-dicarboxylic acid. Hydrogen ion from the alcohol and hydroxyl ion from the acid react to form water molecules along with Terylene polymer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




