
Which of the following is not a condensation polymer?
(A)- Nylon-6,6
(B)- PTFE
(C)- Dacron
(D)- Glyptal
Answer
588.6k+ views
Hint: Polymerization is a process of combining or adding two or more monomers through chemical reactions forming long polymer chains or three-dimensional structures. The basic fundamental unit of polymers which reacts with another fundamental unit to form a bigger molecule is known as a monomer.
Complete step by step answer:
-There are two basic types of polymerization, that is addition polymerization and condensation polymerization.
-Condensation polymerization- When repetitive condensation between two bi-functional monomers occurs, then the polymers are said to undergo condensation polymerization. In condensation polymerization, loss of simple molecules like water and alcohols are formed as the by-products. The mechanism of condensation polymerization refers to the combining of small molecules to obtain larger molecules and the product formed in every step is a bi-functional species. Condensation polymerization is also known as step-reaction polymerization.
-The characteristics of condensation polymerization reactions are as follows-
(i) The monomers must have one or two functional groups, like alcohol, amine, or carboxylic groups.
(ii) The two monomers can be either of similar or of two different functional group monomers. It can also take place between a dimer and an oligomer, one monomer, and one diner or between a chain or another chain of polymers.
(iii) Monomers are combined to form a larger polymer unit.
(iv) Mixed properties of both functional groups of the monomers or molecules are taken into consideration.
(v) Condensation polymer gives a linear polymer as the product.
(vi) When one of the functional groups of the monomer is trifunctional or tetra functional, then the polymer formed will be a cross-linked polymer having a three-dimensional network.
(viii) When the monomer with one reactive group is added, the average molecular weight decreases.
-Examples of molecules that undergo condensation polymers are Nylon-6, Dacron (or terylene) and Glyptal, etc.
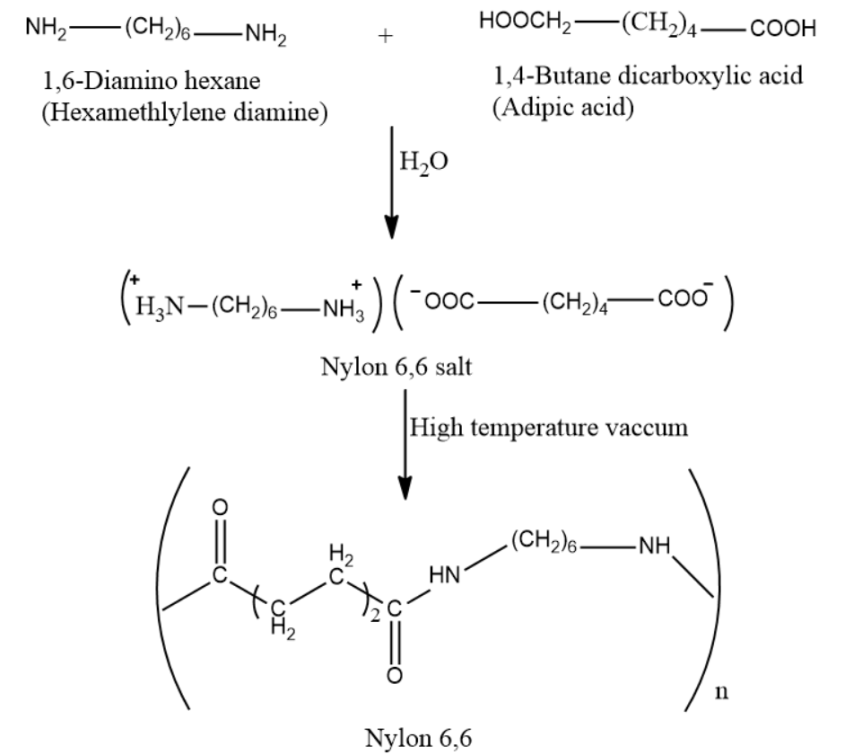
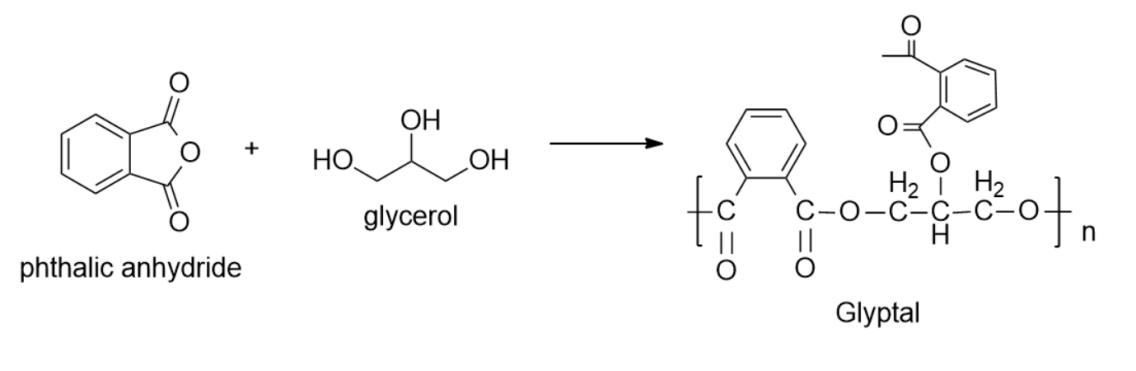
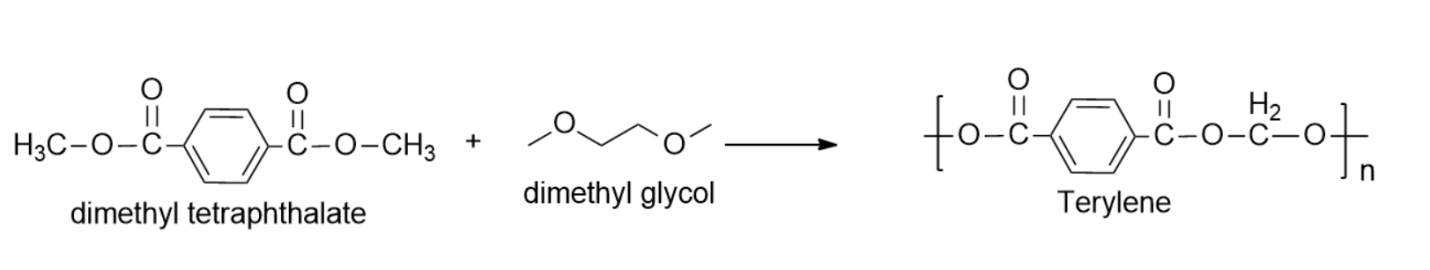
So, the correct answer is “Option B”.
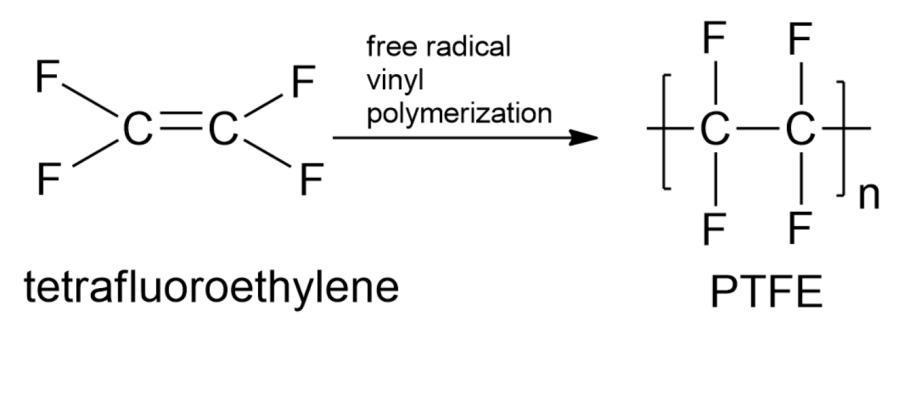
Note: Another type of condensation is the addition of polymerization. Addition polymerization is one of the most common types of reactions forming polymers is chain-reaction of addition polymerization. This polymerization is a three-step reaction involving two chemical moieties. When we keep on adding monomers one after another, we will obtain a large chain of polymers, and such a process is called the chain-growth polymerization. Here the monomers used are unsaturated compounds that connect by each other by double or triple covalent bonds. Example, Polytetrafluoroethylene (PTFE).
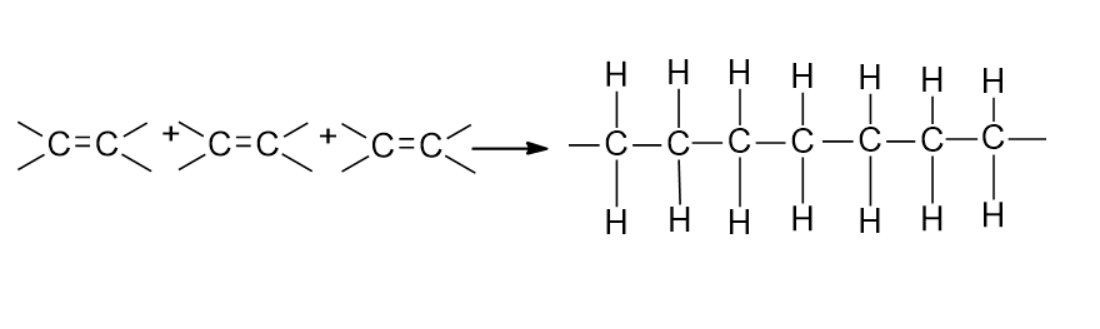
Complete step by step answer:
-There are two basic types of polymerization, that is addition polymerization and condensation polymerization.
-Condensation polymerization- When repetitive condensation between two bi-functional monomers occurs, then the polymers are said to undergo condensation polymerization. In condensation polymerization, loss of simple molecules like water and alcohols are formed as the by-products. The mechanism of condensation polymerization refers to the combining of small molecules to obtain larger molecules and the product formed in every step is a bi-functional species. Condensation polymerization is also known as step-reaction polymerization.
-The characteristics of condensation polymerization reactions are as follows-
(i) The monomers must have one or two functional groups, like alcohol, amine, or carboxylic groups.
(ii) The two monomers can be either of similar or of two different functional group monomers. It can also take place between a dimer and an oligomer, one monomer, and one diner or between a chain or another chain of polymers.
(iii) Monomers are combined to form a larger polymer unit.
(iv) Mixed properties of both functional groups of the monomers or molecules are taken into consideration.
(v) Condensation polymer gives a linear polymer as the product.
(vi) When one of the functional groups of the monomer is trifunctional or tetra functional, then the polymer formed will be a cross-linked polymer having a three-dimensional network.
(viii) When the monomer with one reactive group is added, the average molecular weight decreases.
-Examples of molecules that undergo condensation polymers are Nylon-6, Dacron (or terylene) and Glyptal, etc.



So, the correct answer is “Option B”.
Note: Another type of condensation is the addition of polymerization. Addition polymerization is one of the most common types of reactions forming polymers is chain-reaction of addition polymerization. This polymerization is a three-step reaction involving two chemical moieties. When we keep on adding monomers one after another, we will obtain a large chain of polymers, and such a process is called the chain-growth polymerization. Here the monomers used are unsaturated compounds that connect by each other by double or triple covalent bonds. Example, Polytetrafluoroethylene (PTFE).


Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




