
Which of the following is known as Freon?
A.$CC{l_2}{F_2}$
B.$CHC{l_3}$
C.$C{H_2}{F_2}$
D.$C{F_4}$
Answer
517.8k+ views
Hint: We have to know that Freon’s are chlorofluorocarbons that are considered as significant poly-halogens. When we replace the atoms of hydrogen in methane with chlorine and fluorine, we obtain chlorofluorocarbons. Based on the number of chlorine and fluorine atoms, the properties of chlorofluorocarbons vary.
Complete answer:
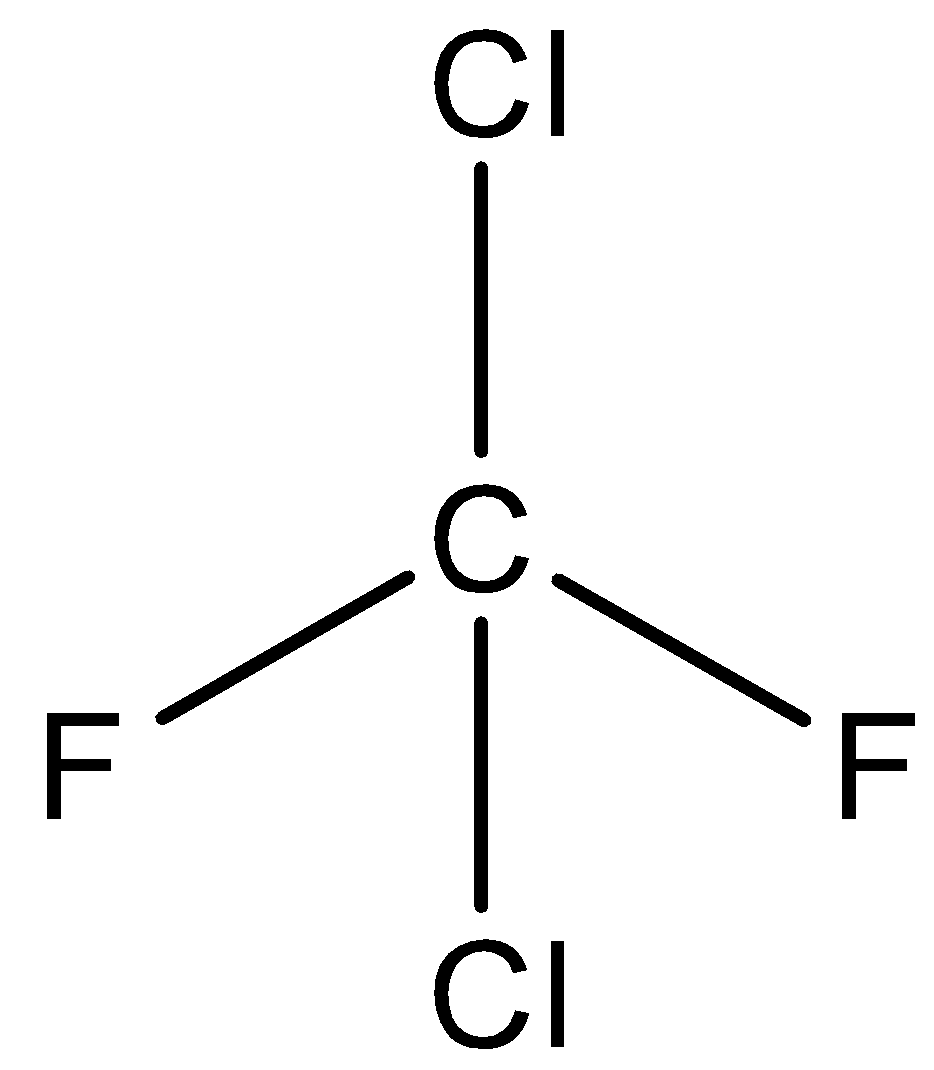
We could say that the chlorofluoro compounds of methane and ethane where all atoms of hydrogen get substituted by halogen atoms are known as Freon.
We have to know that among the given options, dichlorodifluoromethane (or) difluorodichloromethane is hydro chlorofluorocarbon (HCFC). When the hydrogens found in molecules of methane are replaced by two atoms of chlorine and two atoms of fluorine, a molecule of Freon taking the symbol $CC{l_2}{F_2}$ is obtained.
We can give the structure of Freon as,
We know that Freon is odorless, non-toxic gas, and non-corrosive. They are stable at high pressures and volumes. Freon is used as a force and refrigerant. These applications are getting removed in certain nations due to the ozone consumption potential of the compound. Freons are non-toxic when they are utilized in perfumes, aerosols, sprays etc. Their manufacture and use as refrigerants could have decreased these days because they are not very eco-friendly.
Among the following options, a Freon is $CC{l_2}{F_2}$.
Option (A) is correct.
Note:
We have to know that it’s because dichlorodifluoromethane, depletion in ozone is caused. Trichlorofluoromethane and chlorodifluoromethane are some other examples of chlorofluorocarbons. We can write that the chemical representation of Trichlorofluoromethane is $CC{l_3}F$. The chemical representation of chlorodifluoromethane is $CHCl{F_2}$.
Complete answer:
We could say that the chlorofluoro compounds of methane and ethane where all atoms of hydrogen get substituted by halogen atoms are known as Freon.
We have to know that among the given options, dichlorodifluoromethane (or) difluorodichloromethane is hydro chlorofluorocarbon (HCFC). When the hydrogens found in molecules of methane are replaced by two atoms of chlorine and two atoms of fluorine, a molecule of Freon taking the symbol $CC{l_2}{F_2}$ is obtained.
We can give the structure of Freon as,

We know that Freon is odorless, non-toxic gas, and non-corrosive. They are stable at high pressures and volumes. Freon is used as a force and refrigerant. These applications are getting removed in certain nations due to the ozone consumption potential of the compound. Freons are non-toxic when they are utilized in perfumes, aerosols, sprays etc. Their manufacture and use as refrigerants could have decreased these days because they are not very eco-friendly.
Among the following options, a Freon is $CC{l_2}{F_2}$.
Option (A) is correct.
Note:
We have to know that it’s because dichlorodifluoromethane, depletion in ozone is caused. Trichlorofluoromethane and chlorodifluoromethane are some other examples of chlorofluorocarbons. We can write that the chemical representation of Trichlorofluoromethane is $CC{l_3}F$. The chemical representation of chlorodifluoromethane is $CHCl{F_2}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




