
Which of the following is an electrophile?
A. \[{\text{:CC}}{{\text{l}}_{\text{2}}}\]
B. \[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\]
C. \[{{\text{H}}_{\text{2}}}{\text{O}}\]
D. \[{\text{N}}{{\text{H}}_{\text{3}}}\]
Answer
569.7k+ views
Hint:Electrophile is an electron-deficient species. It tends to accept electrons. Determine the species that has less number of electrons.
Complete answer:
An electrophile is a species having an electron-deficient atom or center. Due to the deficiency of electrons, they tend to gain electrons.
A. The species given in option A is\[{\text{:CC}}{{\text{l}}_{\text{2}}}\]. It is dichlorocarbene. The structure of \[{\text{:CC}}{{\text{l}}_{\text{2}}}\] is as follows:
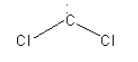
Here, the central carbon atom is surrounded by 6 electrons so we can say that it is an electron-deficient species. So, dichlorocarbene \[{\text{:CC}}{{\text{l}}_{\text{2}}}\] is an electrophile.
Thus, option (A) \[{\text{:CC}}{{\text{l}}_{\text{2}}}\] is correct.
B. The species given in option B is\[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\]. The structure of \[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\] is as follows:
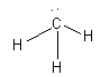
Here, the central carbon atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider \[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\] as an electrophile.
Thus, option (B) \[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\] is incorrect.
C. The species given in option C is\[{{\text{H}}_{\text{2}}}{\text{O}}\]. The structure of \[{{\text{H}}_{\text{2}}}{\text{O}}\] is as follows:
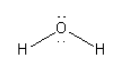
Here, the central oxygen atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider \[{{\text{H}}_{\text{2}}}{\text{O}}\] as an electrophile.
Thus, option (C) \[{{\text{H}}_{\text{2}}}{\text{O}}\] is incorrect.
D. The species given in option D is\[{\text{N}}{{\text{H}}_{\text{3}}}\]. The structure of \[{\text{N}}{{\text{H}}_{\text{3}}}\] is as follows:
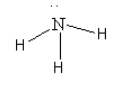
Here, the central nitrogen atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider \[{\text{N}}{{\text{H}}_{\text{3}}}\] as an electrophile.
Thus, option (D) \[{\text{N}}{{\text{H}}_{\text{3}}}\]is incorrect.
Hence, the correct option is (A) \[{\text{:CC}}{{\text{l}}_{\text{2}}}\].
Note:
Carbene are neutral species having a carbon atom with two bonds. In carbene central carbon atoms are surrounded by 6 electrons. As the octet of central carbon in carbene is incomplete they are known as electrophile.
Complete answer:
An electrophile is a species having an electron-deficient atom or center. Due to the deficiency of electrons, they tend to gain electrons.
A. The species given in option A is\[{\text{:CC}}{{\text{l}}_{\text{2}}}\]. It is dichlorocarbene. The structure of \[{\text{:CC}}{{\text{l}}_{\text{2}}}\] is as follows:

Here, the central carbon atom is surrounded by 6 electrons so we can say that it is an electron-deficient species. So, dichlorocarbene \[{\text{:CC}}{{\text{l}}_{\text{2}}}\] is an electrophile.
Thus, option (A) \[{\text{:CC}}{{\text{l}}_{\text{2}}}\] is correct.
B. The species given in option B is\[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\]. The structure of \[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\] is as follows:

Here, the central carbon atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider \[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\] as an electrophile.
Thus, option (B) \[{\text{C}}{{\text{H}}_{\text{3}}}^{\text{ - }}\] is incorrect.
C. The species given in option C is\[{{\text{H}}_{\text{2}}}{\text{O}}\]. The structure of \[{{\text{H}}_{\text{2}}}{\text{O}}\] is as follows:

Here, the central oxygen atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider \[{{\text{H}}_{\text{2}}}{\text{O}}\] as an electrophile.
Thus, option (C) \[{{\text{H}}_{\text{2}}}{\text{O}}\] is incorrect.
D. The species given in option D is\[{\text{N}}{{\text{H}}_{\text{3}}}\]. The structure of \[{\text{N}}{{\text{H}}_{\text{3}}}\] is as follows:

Here, the central nitrogen atom is surrounded by 8 electrons so we can say that it is an electron-rich species. So, we cannot consider \[{\text{N}}{{\text{H}}_{\text{3}}}\] as an electrophile.
Thus, option (D) \[{\text{N}}{{\text{H}}_{\text{3}}}\]is incorrect.
Hence, the correct option is (A) \[{\text{:CC}}{{\text{l}}_{\text{2}}}\].
Note:
Carbene are neutral species having a carbon atom with two bonds. In carbene central carbon atoms are surrounded by 6 electrons. As the octet of central carbon in carbene is incomplete they are known as electrophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




