
Which of the following is a transition element?
A. $Al$
B.$As$
C.$Ni$
D.$Rb$
Answer
578.1k+ views
Hint: Transition elements are those elements which are placed between metals and non - metals in periodic table. They are also called d-block elements.
Complete step by step answer:
Let us first explain the periodic table.
According to modern periodic table given by Moslay:
Non - Metals are placed in group \[13\] to group \[17.\]Inert gases are placed in group 18. And transition elements are placed in group 3 to group 12.
Aluminum$(Al)$ belongs to group \[3.\] Therefore, it is metal.
Arsenic$(As)$ belongs to the group \[15\] therefore it is metalloid.
Rubidium $({R_b})$ belongs to group $1$. Therefore, it is metal.
Nickel $(Ni)$ belongs to group $10$, therefore it is transition metal.
So, the correct answer is “Option C”.
Additional information:
The Atomic Number of $Ni$ is $28.$
It’s electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2}.$
$ = [Ne]3{s^2}3{p^6}3{d^8}4{s^2}$.
Since Electronic configuration of $Ne$ is $1{s^2}2{s^2}2{p^6}$. Therefore, we can write $Ne$ in place of $1{s^2}2{s^2}2{p^6}$.
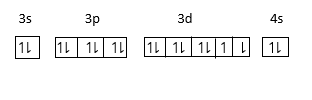
The energy of $4s$ orbital is less than $3d$ orbital therefore electron enters in $4s$ orbital first and then $3d$ orbital therefore electron enters in us first and then $3d.$ Thus last electron in Nickel enters in $d - $ orbital. Therefore, it is known as $d - block$ elements.
Note:
There are four transition series called as first (4d), second (5d), third (6d) and Fourth (7d). Nickel belongs to the first transition series.
Transition element can be identified by filling electrons in the orbital. If the last electron enters in $d - $orbital it is a transition element. Electrons should be filled according to Aufbau principle.
Complete step by step answer:
Let us first explain the periodic table.
According to modern periodic table given by Moslay:
Non - Metals are placed in group \[13\] to group \[17.\]Inert gases are placed in group 18. And transition elements are placed in group 3 to group 12.
Aluminum$(Al)$ belongs to group \[3.\] Therefore, it is metal.
Arsenic$(As)$ belongs to the group \[15\] therefore it is metalloid.
Rubidium $({R_b})$ belongs to group $1$. Therefore, it is metal.
Nickel $(Ni)$ belongs to group $10$, therefore it is transition metal.
So, the correct answer is “Option C”.
Additional information:
The Atomic Number of $Ni$ is $28.$
It’s electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^2}.$
$ = [Ne]3{s^2}3{p^6}3{d^8}4{s^2}$.
Since Electronic configuration of $Ne$ is $1{s^2}2{s^2}2{p^6}$. Therefore, we can write $Ne$ in place of $1{s^2}2{s^2}2{p^6}$.

The energy of $4s$ orbital is less than $3d$ orbital therefore electron enters in $4s$ orbital first and then $3d$ orbital therefore electron enters in us first and then $3d.$ Thus last electron in Nickel enters in $d - $ orbital. Therefore, it is known as $d - block$ elements.
Note:
There are four transition series called as first (4d), second (5d), third (6d) and Fourth (7d). Nickel belongs to the first transition series.
Transition element can be identified by filling electrons in the orbital. If the last electron enters in $d - $orbital it is a transition element. Electrons should be filled according to Aufbau principle.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




