
which of the following have non-zero dipole moments?
(A) $ S{F_4} $
(B) $ S{F_6} $
(C) $ Xe{O_2}{F_2} $
(D) $ S{O_3} $
Answer
517.2k+ views
Hint :Dipole moment is the measure of polarity of a chemical bond within a molecule. It occurs whenever there is a separation of positive and negative charges. So, polarity is nothing but a separation of electric charge leading to a molecule having an electric dipole moment.
Complete Step By Step Answer:
The dipole moment is represented by $ \mu $ (mue).
Dipole moment is given by
$ \mu = \delta d $
Where $ \delta $ -charge ( $ {\delta ^ + } $ and $ {\delta ^ - } $ )
d-distance between partial charges.
The unit of $ \mu $ is debye(D).
$ S{F_4} $ molecule – name of the molecule is sulphur tetrafluoride. The hybridization type of the molecule is $ s{p^3}d $ hybridization. The molecular geometry is trigonal bipyramidal. It contains four bond pairs and one lone pair.
It has a see-saw shape.
(all diagrams are drawn using paint 3d)
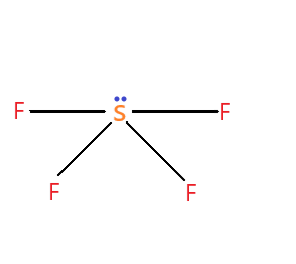
The molecular geometry of the $ S{F_4} $ molecule is not linear or any geometrical shape that will cancel out the partial negative charges on the fluorine, so the overall molecule is polar which makes it have a dipole moment of around 0.632D. So it has a non-zero dipole moment.
$ S{F_6} $ molecule – name of the molecule is sulphur hexafluoride. The molecule have $ s{p^3}{d^2} $ hybridization. The molecular geometry is octahedral.
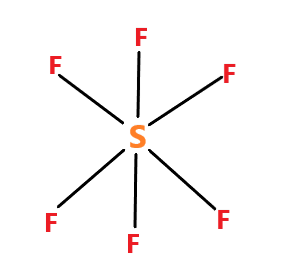
The $ S{F_6} $ molecule has no dipole moment because each $ S - F $ bond dipole is balanced by one of equal magnitude pointing in opposite direction of the other side of the molecule. So the net charge of the molecule is zero.
$ Xe{O_2}{F_2} $ molecule – the molecule name is xenon dioxide difluoride. The molecule have $ s{p^3}d $ hybridization. The molecular geometry is trigonal bipyramidal.
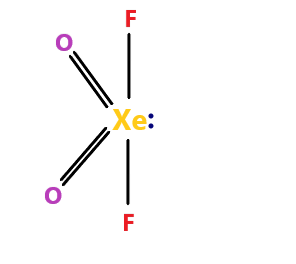
The molecule is polar. It has 4 bond pairs and 1 lone pair. Since this molecule has a lone pair, it is not symmetrical and therefore, has a net polar moment.
$ S{O_3} $ molecule – name of the molecule is sulphur trioxide. The molecule have $ s{p^2} $ hybridization. The molecular geometry is trigonal planar.
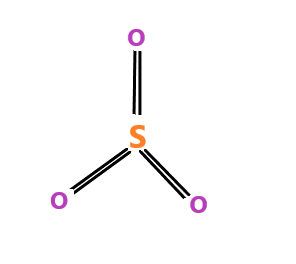
$ S{O_3} $ is a planar molecule with three bond angles 120 degrees apart. The individual dipole moments of $ S - O $ bond cancel out each other due to which the dipole moment of the molecule is zero.
Hence the option (A) and (C) have non zero dipole moments.
Note :
from dipole moment
-Ionic characters can be calculated.
-Geometry of the molecule can be predicted.
-Nature of the molecule can be predicted.
Keep in point that the dipole moment is a vector quantity.
Complete Step By Step Answer:
The dipole moment is represented by $ \mu $ (mue).
Dipole moment is given by
$ \mu = \delta d $
Where $ \delta $ -charge ( $ {\delta ^ + } $ and $ {\delta ^ - } $ )
d-distance between partial charges.
The unit of $ \mu $ is debye(D).
$ S{F_4} $ molecule – name of the molecule is sulphur tetrafluoride. The hybridization type of the molecule is $ s{p^3}d $ hybridization. The molecular geometry is trigonal bipyramidal. It contains four bond pairs and one lone pair.
It has a see-saw shape.
(all diagrams are drawn using paint 3d)

The molecular geometry of the $ S{F_4} $ molecule is not linear or any geometrical shape that will cancel out the partial negative charges on the fluorine, so the overall molecule is polar which makes it have a dipole moment of around 0.632D. So it has a non-zero dipole moment.
$ S{F_6} $ molecule – name of the molecule is sulphur hexafluoride. The molecule have $ s{p^3}{d^2} $ hybridization. The molecular geometry is octahedral.

The $ S{F_6} $ molecule has no dipole moment because each $ S - F $ bond dipole is balanced by one of equal magnitude pointing in opposite direction of the other side of the molecule. So the net charge of the molecule is zero.
$ Xe{O_2}{F_2} $ molecule – the molecule name is xenon dioxide difluoride. The molecule have $ s{p^3}d $ hybridization. The molecular geometry is trigonal bipyramidal.

The molecule is polar. It has 4 bond pairs and 1 lone pair. Since this molecule has a lone pair, it is not symmetrical and therefore, has a net polar moment.
$ S{O_3} $ molecule – name of the molecule is sulphur trioxide. The molecule have $ s{p^2} $ hybridization. The molecular geometry is trigonal planar.

$ S{O_3} $ is a planar molecule with three bond angles 120 degrees apart. The individual dipole moments of $ S - O $ bond cancel out each other due to which the dipole moment of the molecule is zero.
Hence the option (A) and (C) have non zero dipole moments.
Note :
from dipole moment
-Ionic characters can be calculated.
-Geometry of the molecule can be predicted.
-Nature of the molecule can be predicted.
Keep in point that the dipole moment is a vector quantity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




