
Which of the following have –M effect ( \[{e^ - }\] withdrawing effect)?
A.
B.\[ - S{O_3}H\]
C.\[ - OR\]
D.\[ - Br\]

Answer
583.2k+ views
Hint: The electronegativity of an element will have capability to withdraw the electron from the neighbouring atom towards itself.
Complete Step by step answer:
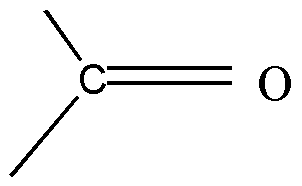
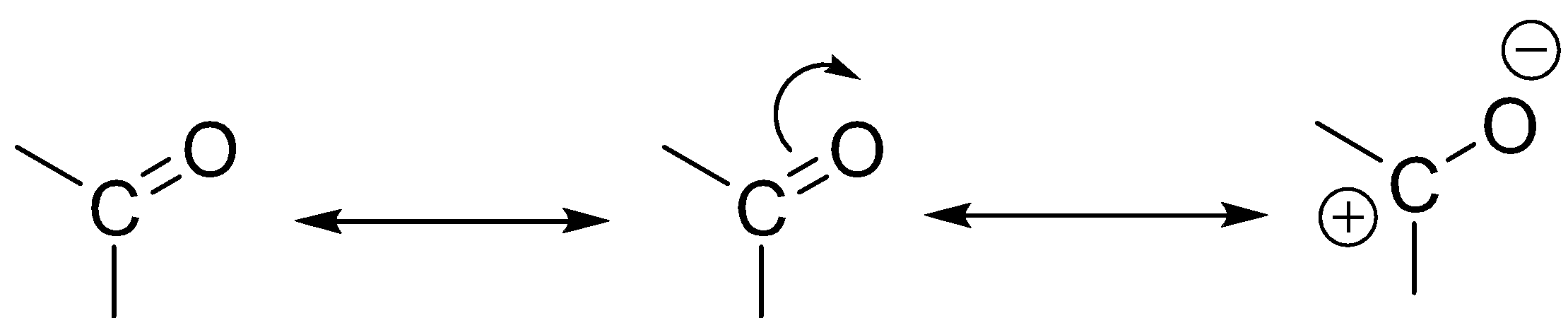
Carbonyl:
The \[C = O\] functional group available in a system will have –M effect as the oxygen atom which is highly electronegative in nature will attract the electron from the \[C - O\] bond and make the carbon electron deficient. They will lead to the withdrawal of electrons from the system where the \[C = O\] is attached with.
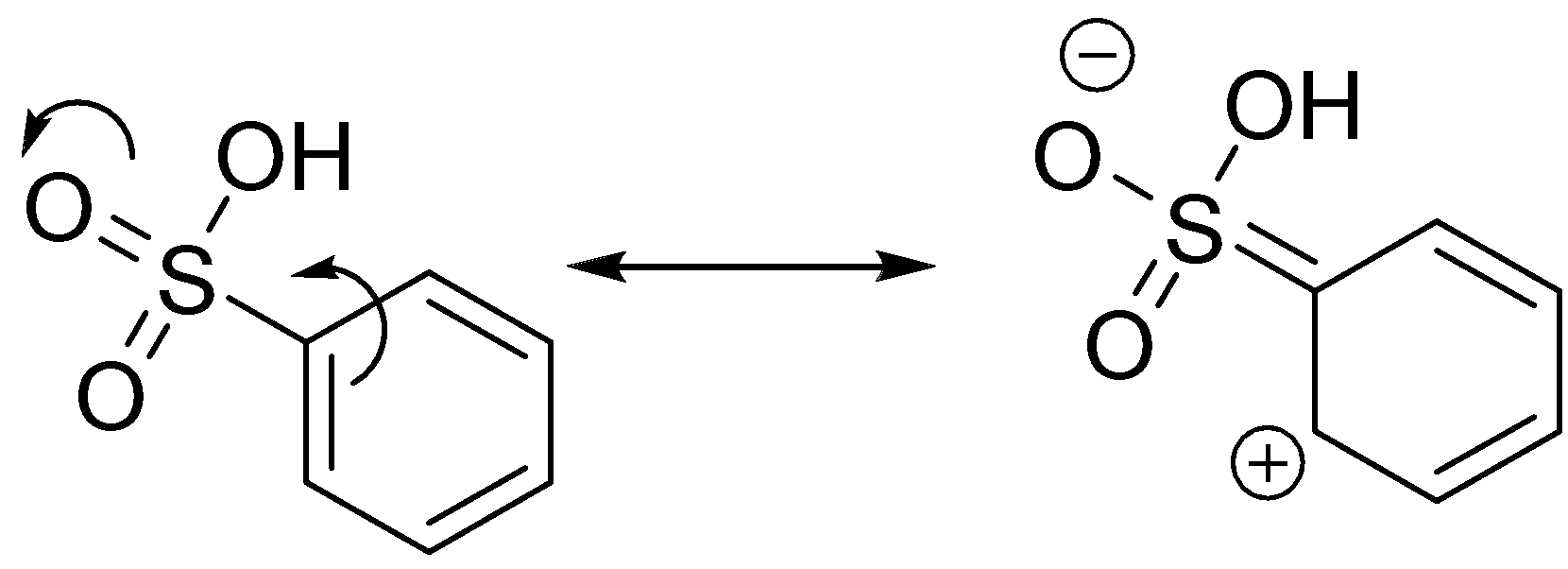
Sulfonic acid:
The sulphur is relatively higher in size than carbon. Similar electron withdrawing effect was observed as the presence of two oxygen atoms connected with sulphur through the pi bond.
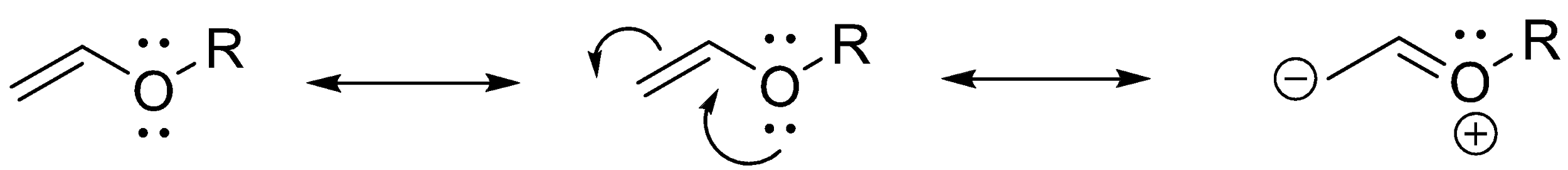
\[ - OR\] :
Alkyl and alkoxides are ready to donate electrons into the system where it is connected and hence, they show +M effect. The lone pair of electrons present in the oxygen will be moved towards the pi bond present in the molecule.
\[ - Br\] :
In general, the halogens are electronegative in nature but the electronegativity reduces when going from top to bottom in the periodic table. Bromine is bigger in size and the effect was very less. Bromine is the least electronegative halogen atom.
Note: Groups like \[C = O\] , \[ - N{O_2}\] , \[ - COOH\] , \[ - COOR\] , \[ - S{O_3}H\] when present adjacent to a multiple bond in molecules are conjugated with the multiple bonds. They bring resonance into the molecule by withdrawing the π electrons and cause electron displacement towards themselves.
When a group showing -M effect is present as a substituent in benzene, it makes the benzene ring less reactive towards electrophilic substitution.
Complete Step by step answer:
Carbonyl:
The \[C = O\] functional group available in a system will have –M effect as the oxygen atom which is highly electronegative in nature will attract the electron from the \[C - O\] bond and make the carbon electron deficient. They will lead to the withdrawal of electrons from the system where the \[C = O\] is attached with.

Sulfonic acid:
The sulphur is relatively higher in size than carbon. Similar electron withdrawing effect was observed as the presence of two oxygen atoms connected with sulphur through the pi bond.

\[ - OR\] :
Alkyl and alkoxides are ready to donate electrons into the system where it is connected and hence, they show +M effect. The lone pair of electrons present in the oxygen will be moved towards the pi bond present in the molecule.

\[ - Br\] :
In general, the halogens are electronegative in nature but the electronegativity reduces when going from top to bottom in the periodic table. Bromine is bigger in size and the effect was very less. Bromine is the least electronegative halogen atom.
Note: Groups like \[C = O\] , \[ - N{O_2}\] , \[ - COOH\] , \[ - COOR\] , \[ - S{O_3}H\] when present adjacent to a multiple bond in molecules are conjugated with the multiple bonds. They bring resonance into the molecule by withdrawing the π electrons and cause electron displacement towards themselves.
When a group showing -M effect is present as a substituent in benzene, it makes the benzene ring less reactive towards electrophilic substitution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




