
Which of the following compounds undergo nucleophilic substitution reaction most easily?
A)
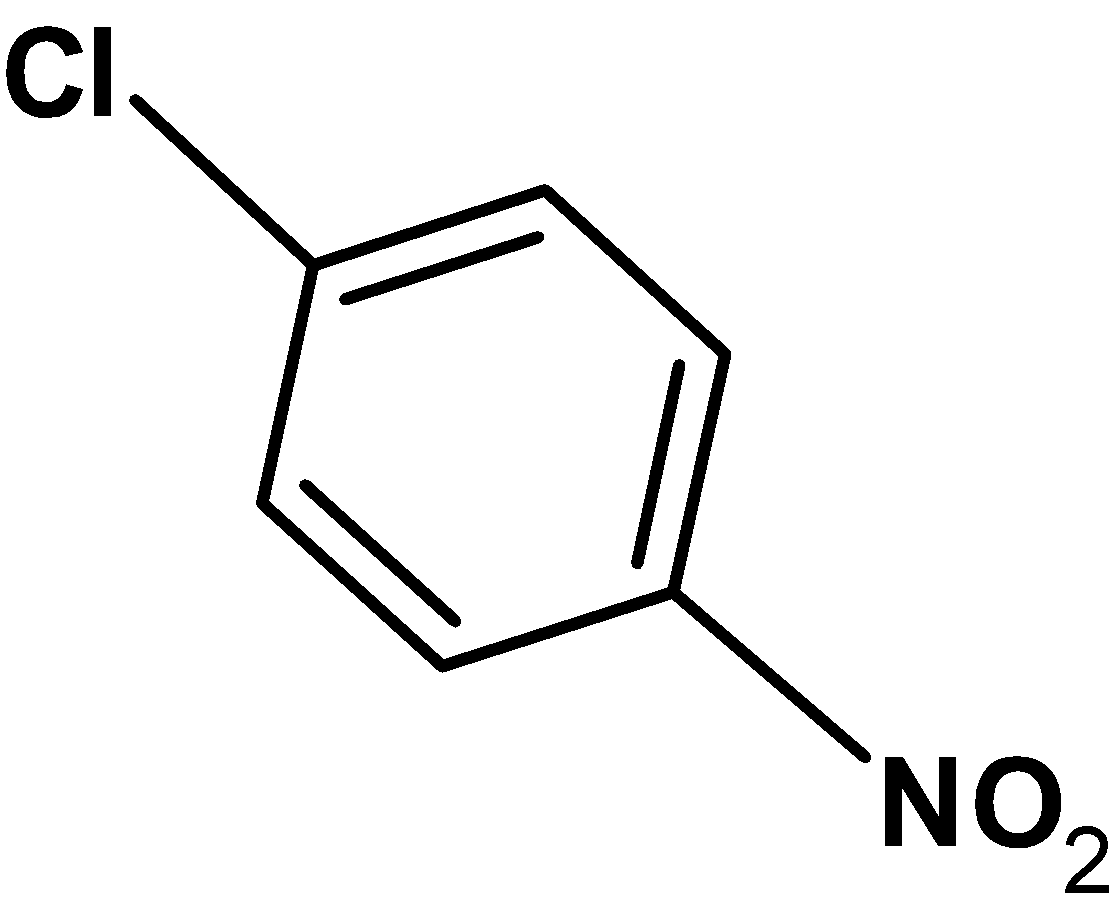
B)
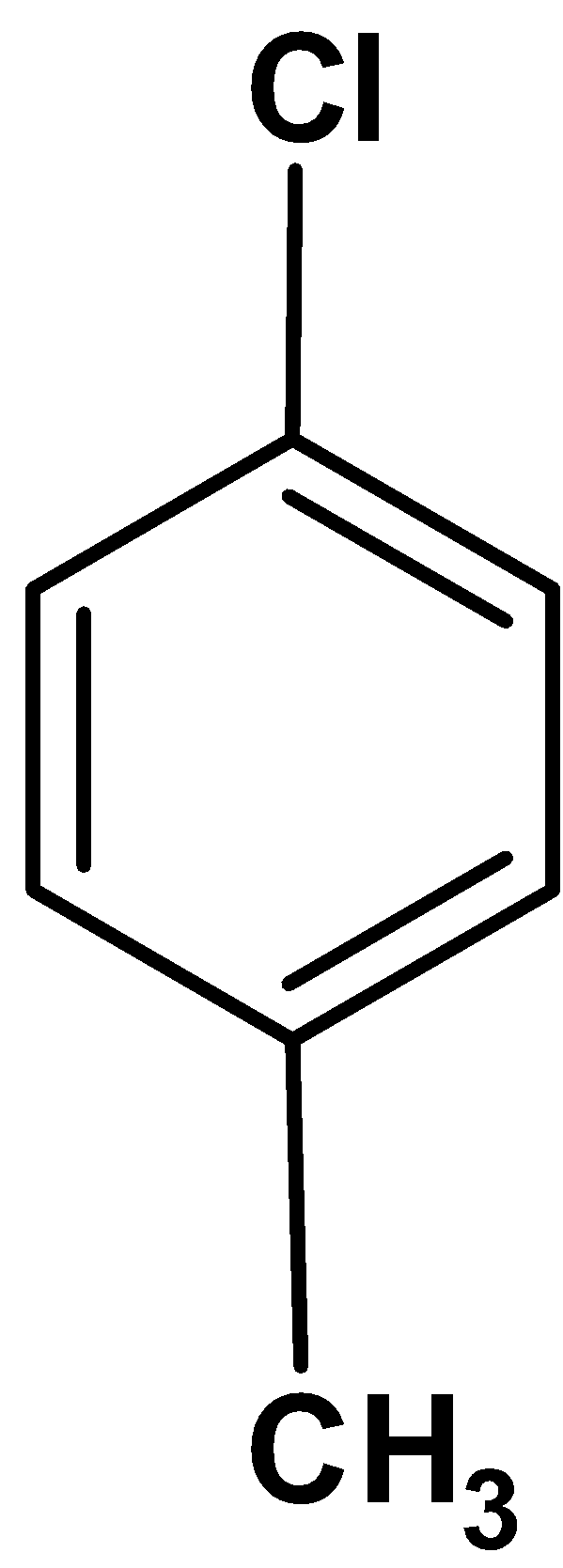
C)
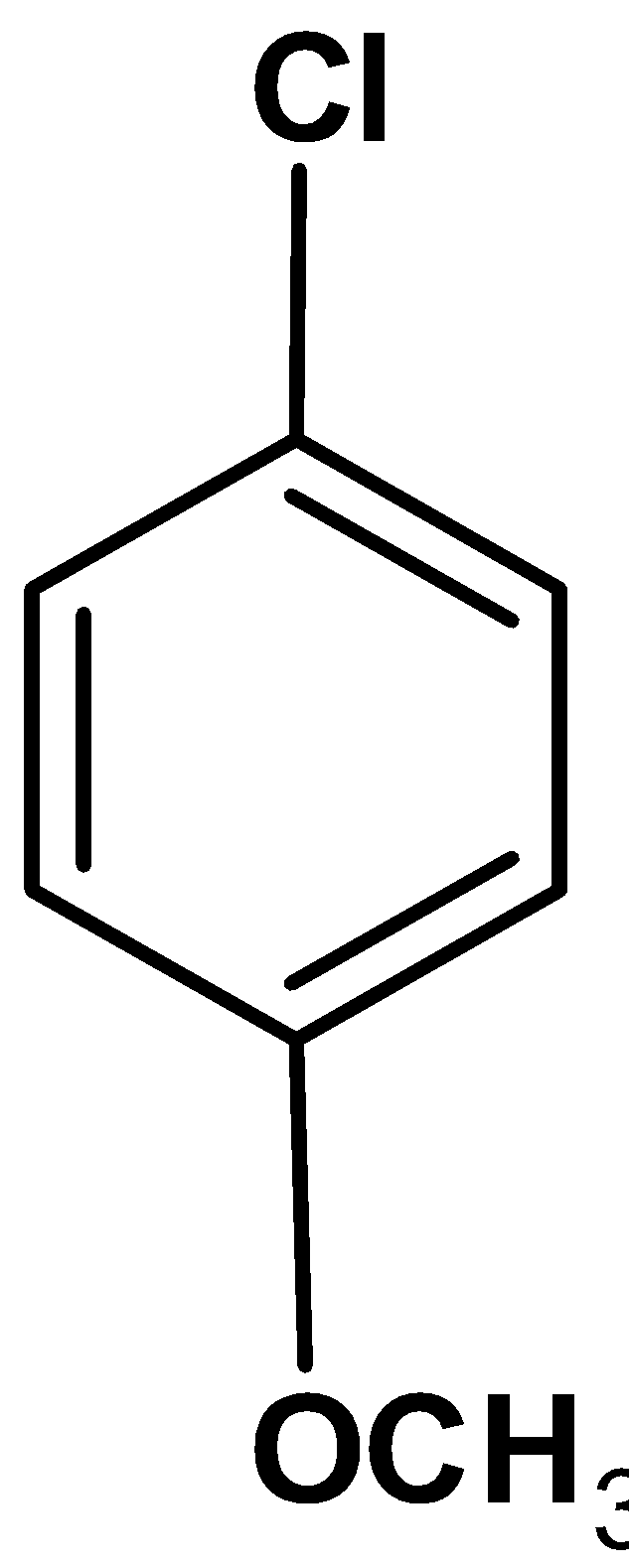
D)
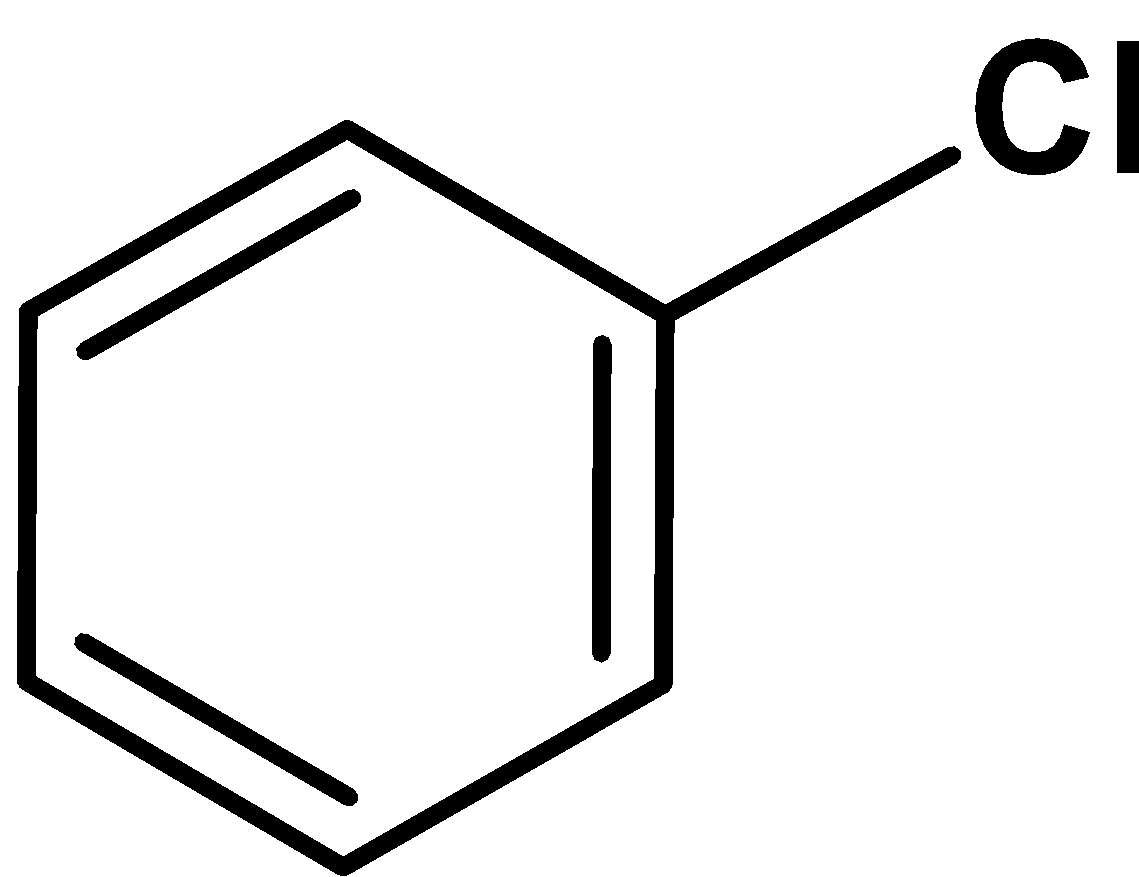
| A) | 
|
| B) | 
|
| C) | 
|
| D) | 
|
Answer
577.8k+ views
Hint: The more reactive nucleophile knocks out the less reactive nucleophile and occupies its position is known as the nucleophilic substitution reaction. The nucleophilic substitution reaction depends on the various factors, like the basicity of nucleophile, electronegativity, substituents on the ring, the strength of nucleophile, etc. The electron-withdrawing or electron-donating groups alters the rate of reaction.
Complete step by step answer:
Nucleophilic substitution reactions are a type of organic reaction. This reaction involves a replacement of a nucleophile by the other. When nucleophiles attack on the carbon by replacing the existing nucleophile which results in the substitution is known as the nucleophilic substitution reaction.
The nucleophilic substitution reaction is affected by the electron-withdrawing or electron-donating groups. The electron-withdrawing group facilitates the substitution reaction but the electron-donating group decreases the nucleophilic attack.
In p-nitro chlorobenzene, the nitro group $\text{ (}-\text{N}{{\text{O}}_{\text{2}}}\text{ ) }$ is an electron-withdrawing group. When at the para position, it results in the extended conjugation such that the electron density is withdrawn to the nitro group. These decrease the electron density on the ring. Thus, the chloride ion $\text{ C}{{\text{l}}^{-}}\text{ }$ and carbon bond (from the ring) weakens .when more reactive nucleophile like$\text{ O}{{\text{H}}^{-}}\text{ }$ attacks on the p-nitro chlorobenzene, it undergoes the nucleophilic substitution reaction easily.
When the aromatic ring is bonded to the electron-withdrawing group, electron density moves towards the electron withdrawing group and the aromatic ring becomes electrophilic and thus nucleophile can easily attack.
When the aromatic ring is bonded to an electron-donating or releasing group, electron density moves towards the aromatic ring thus the aromatic ring becomes nucleophilic and thus nucleophile cannot easily attack on the ring. In such cases, the ring can easily undergo the electrophilic substitution reaction.
Methoxy is an electron-donating group. When bonded to the aromatic ring, it donates its electron density to the ring. This in turns increases the electron density around the nucleophilic groups (existing) on the ring. Because of the high electron density, the existing nucleophile cannot be knocked off easily. Thus, nucleophilic substitution reactions are difficult to carry out in presence of an electron-donating group. The p-methylchlorobenzene does not undergo the nucleophilic substitution reaction easily.
The decreasing order of the ease towards the nucleophilic substitution reaction is as shown below,
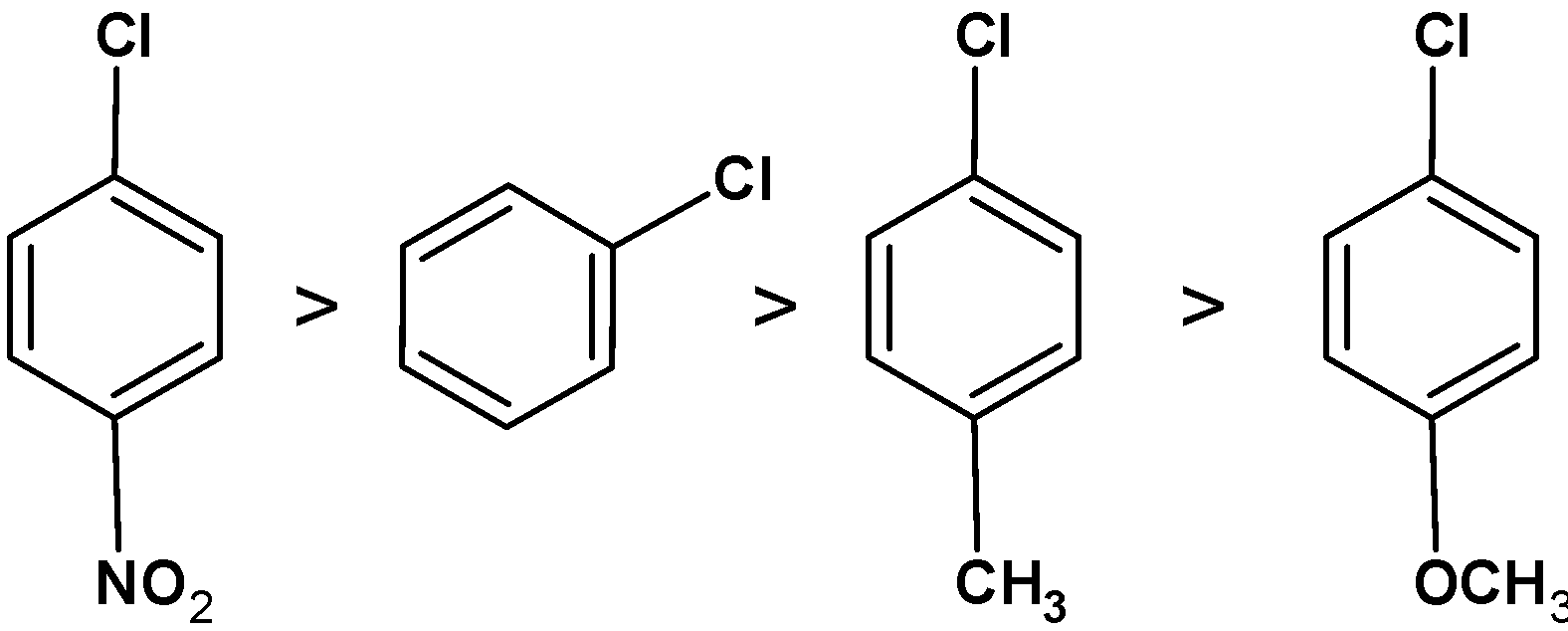
The p-chloronitrobenzene can easily undergo the nucleophilic substitution reaction.
So, the correct answer is “Option A”.
Note: Let's consider an example of the nucleophilic substitution of chlorobenzene. The chlorobenzene is converted into the phenol by the aqueous sodium hydroxide only when the temperature is over $\text{ 30}{{\text{0}}^{\text{0}}}\text{C }$ . But, the presence of nitro groups at o- or p- to the chlorine increases reactivity .o-or p-chloronitrobenzene is converted into nitrophenol on treatment with aq.sodium hydroxide at $\text{ 16}{{\text{0}}^{\text{0}}}\text{C }$. A nitro group at the Meta position does not affect reactivity.
Complete step by step answer:
Nucleophilic substitution reactions are a type of organic reaction. This reaction involves a replacement of a nucleophile by the other. When nucleophiles attack on the carbon by replacing the existing nucleophile which results in the substitution is known as the nucleophilic substitution reaction.
The nucleophilic substitution reaction is affected by the electron-withdrawing or electron-donating groups. The electron-withdrawing group facilitates the substitution reaction but the electron-donating group decreases the nucleophilic attack.
In p-nitro chlorobenzene, the nitro group $\text{ (}-\text{N}{{\text{O}}_{\text{2}}}\text{ ) }$ is an electron-withdrawing group. When at the para position, it results in the extended conjugation such that the electron density is withdrawn to the nitro group. These decrease the electron density on the ring. Thus, the chloride ion $\text{ C}{{\text{l}}^{-}}\text{ }$ and carbon bond (from the ring) weakens .when more reactive nucleophile like$\text{ O}{{\text{H}}^{-}}\text{ }$ attacks on the p-nitro chlorobenzene, it undergoes the nucleophilic substitution reaction easily.
When the aromatic ring is bonded to the electron-withdrawing group, electron density moves towards the electron withdrawing group and the aromatic ring becomes electrophilic and thus nucleophile can easily attack.
When the aromatic ring is bonded to an electron-donating or releasing group, electron density moves towards the aromatic ring thus the aromatic ring becomes nucleophilic and thus nucleophile cannot easily attack on the ring. In such cases, the ring can easily undergo the electrophilic substitution reaction.
Methoxy is an electron-donating group. When bonded to the aromatic ring, it donates its electron density to the ring. This in turns increases the electron density around the nucleophilic groups (existing) on the ring. Because of the high electron density, the existing nucleophile cannot be knocked off easily. Thus, nucleophilic substitution reactions are difficult to carry out in presence of an electron-donating group. The p-methylchlorobenzene does not undergo the nucleophilic substitution reaction easily.
The decreasing order of the ease towards the nucleophilic substitution reaction is as shown below,

The p-chloronitrobenzene can easily undergo the nucleophilic substitution reaction.
So, the correct answer is “Option A”.
Note: Let's consider an example of the nucleophilic substitution of chlorobenzene. The chlorobenzene is converted into the phenol by the aqueous sodium hydroxide only when the temperature is over $\text{ 30}{{\text{0}}^{\text{0}}}\text{C }$ . But, the presence of nitro groups at o- or p- to the chlorine increases reactivity .o-or p-chloronitrobenzene is converted into nitrophenol on treatment with aq.sodium hydroxide at $\text{ 16}{{\text{0}}^{\text{0}}}\text{C }$. A nitro group at the Meta position does not affect reactivity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




