
Which of the following compounds react with $ NaOH $ in water?
(A) $ {C_6}{H_5}OH $
(B) $ {C_6}{H_5}C{H_2}OH $
(C) $ {(C{H_3})_3}COH $
(D) $ {C_2}{H_5}OH $
Answer
520.8k+ views
Hint :Among the given compounds, check which is most acidic and which compound will get stabilized more after losing hydrogen. An acid-base reaction will take place between sodium hydroxide and the phenol.
Complete Step By Step Answer:
Consider the first compound phenol, it reacts with sodium hydroxide in water and loses hydrogen. Removal of hydrogen ions takes place and phenoxide ions are formed. Phenoxide ion is very stable because of resonance. It has more resonating structures. If a species has more resonating structures, then that species will be more stable. The structure of phenoxide ion is shown below:
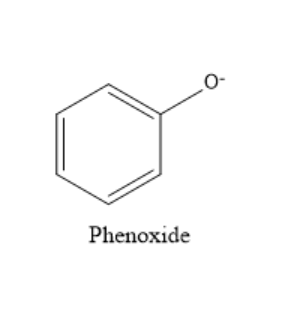
The phenoxide ion is stabilized by resonance. And also observe that in phenoxide ion more electronegative species contain negative charge which makes it even more stable. Consider the compound in the second option. After the removal of hydrogen, there are no possible resonating structures. Similarly, no resonating structures are possible for the other compounds after the removal of hydrogen. So, phenol is the most acidic.
Therefore, option A is the correct answer.
Note :
Phenol is the most acidic because after losing hydrogen, the negative charge generated is stabilized by resonance structures and the energy released in resonance stabilization is higher than those due to inductive effect or any other effects. So, phenolic anion is the most stable. There is an acid-base reaction between phenol and sodium hydroxide in water.
Complete Step By Step Answer:
Consider the first compound phenol, it reacts with sodium hydroxide in water and loses hydrogen. Removal of hydrogen ions takes place and phenoxide ions are formed. Phenoxide ion is very stable because of resonance. It has more resonating structures. If a species has more resonating structures, then that species will be more stable. The structure of phenoxide ion is shown below:

The phenoxide ion is stabilized by resonance. And also observe that in phenoxide ion more electronegative species contain negative charge which makes it even more stable. Consider the compound in the second option. After the removal of hydrogen, there are no possible resonating structures. Similarly, no resonating structures are possible for the other compounds after the removal of hydrogen. So, phenol is the most acidic.
Therefore, option A is the correct answer.
Note :
Phenol is the most acidic because after losing hydrogen, the negative charge generated is stabilized by resonance structures and the energy released in resonance stabilization is higher than those due to inductive effect or any other effects. So, phenolic anion is the most stable. There is an acid-base reaction between phenol and sodium hydroxide in water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




