
Which of the following compounds is most acidic?
(A)
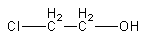
(B)
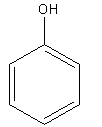
(C)
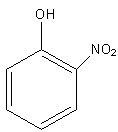
(D)
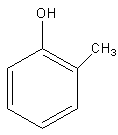
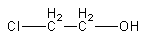



Answer
558.9k+ views
Hint: To determine the acidic compound first we should know what is acidic compounds and how to check the acidic strength of the compound. The compound which donates protons is known as acid. If after the donation of a proton the formed anion is stabilized by any factor then the compound will be acidic. If not the compound will be not acidic or we can say less acidic.
Complete step-by-step answer:We will remove one proton from each of the given compounds and will form their anion then we will check the stabilizing factor for the anion.
More stable the formed anion will cause easy loss of proton so, more will be acidity.
The anion of each compound is shown as follows:
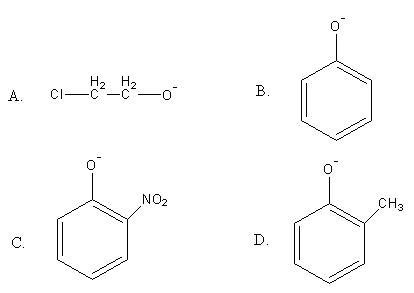
In compound A, the electron-withdrawing negative inductive effect of chlorine is working. The chlorine withdraws some negative charge and stabilizes the anion.
In compound B, the negative charge is delocalised in the ring and stabilised.
In compound C, the negative charge is delocalised in the ring and gets stabilised and compound C also has one nitro group which shows the –M effect. Nitro group is an electron withdrawing group that withdraws some electron density from the negatively charged oxygen hence stabilizing the anion.
In compound D, the negative charge is delocalised in the ring and gets stabilised and compound D also has one methyl group which shows the +I effect. Methyl group is an electron donating group, so it increases the electron density on oxygen hence destabilizing the anion.
So, anions of compound A, B and C are stable, so they will be acidic.Now, the resonance or mesomeric effect is stronger than the inductive effect, so the compound B and C will be more acidic than compound A.In compound B, the negative charge is stabilised only by the delocalization in the ring whereas in compound C with delocalization, -M effect of nitro group is also working, so anion of compound is more stabilised then anion of compound B.So, compound C is more acidic than compound B.So, compound C is most acidic.
Therefore, compound C is the correct answer.
Note:The acids forms anion so, we always check the stability of anion to determine the acidity. The electron-withdrawing (-M) resonance and (-I) inductive effect stabilised the anion so, increases the acidity. The electron-donating (+M) resonance and (+I) inductive effect destabilised the anion so, decreases the acidity. The compound forming aromatic anions is more acidic than the compound forming anti-aromatic anion.
Complete step-by-step answer:We will remove one proton from each of the given compounds and will form their anion then we will check the stabilizing factor for the anion.
More stable the formed anion will cause easy loss of proton so, more will be acidity.
The anion of each compound is shown as follows:

In compound A, the electron-withdrawing negative inductive effect of chlorine is working. The chlorine withdraws some negative charge and stabilizes the anion.
In compound B, the negative charge is delocalised in the ring and stabilised.
In compound C, the negative charge is delocalised in the ring and gets stabilised and compound C also has one nitro group which shows the –M effect. Nitro group is an electron withdrawing group that withdraws some electron density from the negatively charged oxygen hence stabilizing the anion.
In compound D, the negative charge is delocalised in the ring and gets stabilised and compound D also has one methyl group which shows the +I effect. Methyl group is an electron donating group, so it increases the electron density on oxygen hence destabilizing the anion.
So, anions of compound A, B and C are stable, so they will be acidic.Now, the resonance or mesomeric effect is stronger than the inductive effect, so the compound B and C will be more acidic than compound A.In compound B, the negative charge is stabilised only by the delocalization in the ring whereas in compound C with delocalization, -M effect of nitro group is also working, so anion of compound is more stabilised then anion of compound B.So, compound C is more acidic than compound B.So, compound C is most acidic.
Therefore, compound C is the correct answer.
Note:The acids forms anion so, we always check the stability of anion to determine the acidity. The electron-withdrawing (-M) resonance and (-I) inductive effect stabilised the anion so, increases the acidity. The electron-donating (+M) resonance and (+I) inductive effect destabilised the anion so, decreases the acidity. The compound forming aromatic anions is more acidic than the compound forming anti-aromatic anion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




