
Which of the following compounds are called unsaturated compounds- that is, it/they add ${{H}_{2}}$ catalytically at ordinary temperature?
(A)
(B)
(C)
(D)



Answer
590.4k+ views
Hint: Unsaturation can be due to many reasons and not just because of double bond. Apply the formula given below the degree of unsaturation in the above compounds.
Formula: $D.U=\dfrac{2C+2+N-H-X}{2}$
Where,
C = Number of carbon atoms,
N = Number of nitrogen atoms,
H= Number of hydrogen atoms,
X = Number of halogen atoms.
Complete step by step answer:
Degree of Unsaturation also referred to as the index of hydrogen deficiency(IHD) is a measure of the number of rings and pi bonds present in the organic molecule.
However, unsaturation can be due to the presence of atoms like oxygen, nitrogen as well.
We will now calculate the degree of unsaturation for the options and also find whether the molecule is unsaturated or not.
Degree of unsaturation for option (A):
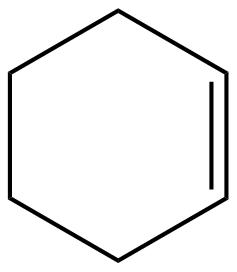
$D.U=\dfrac{12+2-10}{2}=2$
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 2.
Degree of unsaturation for option (B):
$D.U=\dfrac{6+2-6}{2}=1$
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
Degree of unsaturation for option (C):
$D.U=\dfrac{8+2-8}{2}=1$
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
Degree of unsaturation for option (D):
$D.U=\dfrac{10+2-10}{2}=1$
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
So, the correct answer is “Option A,B,C and D”.
Note: Unsaturation in an organic molecule can be due to the following reasons:
-Presence of a double bond (D.U=1)
-Presence of a triple bond (D.U=2)
-Presence of a C=O bond (D.U=1)
-Presence of a C=N bond (D.U=1)
-Presence of a ring (D.U=1)
Formula: $D.U=\dfrac{2C+2+N-H-X}{2}$
Where,
C = Number of carbon atoms,
N = Number of nitrogen atoms,
H= Number of hydrogen atoms,
X = Number of halogen atoms.
Complete step by step answer:
Degree of Unsaturation also referred to as the index of hydrogen deficiency(IHD) is a measure of the number of rings and pi bonds present in the organic molecule.
However, unsaturation can be due to the presence of atoms like oxygen, nitrogen as well.
We will now calculate the degree of unsaturation for the options and also find whether the molecule is unsaturated or not.
Degree of unsaturation for option (A):

$D.U=\dfrac{12+2-10}{2}=2$
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 2.
Degree of unsaturation for option (B):

$D.U=\dfrac{6+2-6}{2}=1$
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
Degree of unsaturation for option (C):

$D.U=\dfrac{8+2-8}{2}=1$
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
Degree of unsaturation for option (D):

$D.U=\dfrac{10+2-10}{2}=1$
Hence the organic molecule is unsaturated with a degree of unsaturation(D.U) = 1.
So, the correct answer is “Option A,B,C and D”.
Note: Unsaturation in an organic molecule can be due to the following reasons:
-Presence of a double bond (D.U=1)
-Presence of a triple bond (D.U=2)
-Presence of a C=O bond (D.U=1)
-Presence of a C=N bond (D.U=1)
-Presence of a ring (D.U=1)
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




