
Which of the following compound have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage where ‘${\text{X}}$’ is the so called central atom like ${\text{P}}$, ${\text{S}}$ etc?
(A) ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$
(B) ${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$
(C) $\gamma - {\text{S}}{{\text{O}}_3}$
(D) ${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$
Answer
573.9k+ views
Hint: To solve this we must first draw the structures of all the four given compounds. From the structures we can determine the compound having ${\text{X}} - {\text{O}} - {\text{X}}$ linkage. First of all we should draw the exact structure of the compound to find out the central atom.
Complete Step by step answer: ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$ is known as peroxydisulfate ion. Draw the structure of ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$:
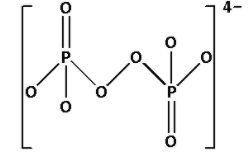
In the structure of ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$, we cannot see ${\text{P}} - {\text{O}} - {\text{P}}$ linkage. Thus, ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$ does not have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage.
Thus, option (A) is not correct.
${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$ is known as thiosulphate ion. Draw the structure of ${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$:
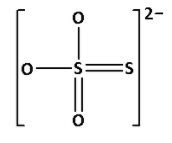
In the structure of ${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$, we cannot see ${\text{S}} - {\text{O}} - {\text{S}}$ linkage. Thus, ${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$ does not have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage.
Thus, option (B) is not correct.
$\gamma - {\text{S}}{{\text{O}}_3}$ is known as sulphur trioxide. Draw the structure of $\gamma - {\text{S}}{{\text{O}}_3}$:
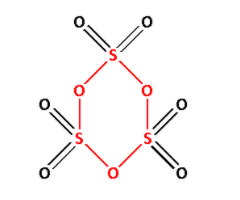
In the structure of $\gamma - {\text{S}}{{\text{O}}_3}$, we can see ${\text{S}} - {\text{O}} - {\text{S}}$ linkage. The ${\text{S}} - {\text{O}} - {\text{S}}$ is shown in red colour. Thus, $\gamma - {\text{S}}{{\text{O}}_3}$ has ${\text{X}} - {\text{O}} - {\text{X}}$ linkage.
Thus, option (C) is correct.
${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$ is known as metabisulphite. Draw the structure of ${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$:
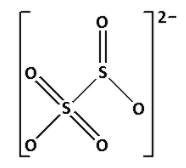
In the structure of ${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$, we cannot see ${\text{S}} - {\text{O}} - {\text{S}}$ linkage. Thus, ${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$ does not have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage.
Thus, option (D) is not correct.
Thus, the compound have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage where ‘${\text{X}}$’ is the so called central atom like ${\text{P}}$, ${\text{S}}$ etc is $\gamma - {\text{S}}{{\text{O}}_3}$.
Thus, the correct option is (C) $\gamma - {\text{S}}{{\text{O}}_3}$.
Note: Sulphur trioxide $\left( {{\text{S}}{{\text{O}}_3}} \right)$ has many structural changes and thus, it has many different forms. These changes are caused by the traces of water. $\gamma - {\text{S}}{{\text{O}}_3}$ is very pure gaseous sulphur trioxide. It is a trimeric structure i.e. it has three molecules of sulphur trioxide. $\gamma - {\text{S}}{{\text{O}}_3}$ forms a cyclic structure. It is colourless solid in appearance. Sulphur trioxide is highly hygroscopic in nature i.e. it can easily attract water molecules from its surroundings by absorption or adsorption.
Complete Step by step answer: ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$ is known as peroxydisulfate ion. Draw the structure of ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$:

In the structure of ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$, we cannot see ${\text{P}} - {\text{O}} - {\text{P}}$ linkage. Thus, ${{\text{P}}_{\text{2}}}{\text{O}}_8^{4 - }$ does not have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage.
Thus, option (A) is not correct.
${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$ is known as thiosulphate ion. Draw the structure of ${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$:

In the structure of ${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$, we cannot see ${\text{S}} - {\text{O}} - {\text{S}}$ linkage. Thus, ${{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }$ does not have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage.
Thus, option (B) is not correct.
$\gamma - {\text{S}}{{\text{O}}_3}$ is known as sulphur trioxide. Draw the structure of $\gamma - {\text{S}}{{\text{O}}_3}$:

In the structure of $\gamma - {\text{S}}{{\text{O}}_3}$, we can see ${\text{S}} - {\text{O}} - {\text{S}}$ linkage. The ${\text{S}} - {\text{O}} - {\text{S}}$ is shown in red colour. Thus, $\gamma - {\text{S}}{{\text{O}}_3}$ has ${\text{X}} - {\text{O}} - {\text{X}}$ linkage.
Thus, option (C) is correct.
${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$ is known as metabisulphite. Draw the structure of ${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$:

In the structure of ${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$, we cannot see ${\text{S}} - {\text{O}} - {\text{S}}$ linkage. Thus, ${{\text{S}}_{\text{2}}}{\text{O}}_5^{2 - }$ does not have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage.
Thus, option (D) is not correct.
Thus, the compound have ${\text{X}} - {\text{O}} - {\text{X}}$ linkage where ‘${\text{X}}$’ is the so called central atom like ${\text{P}}$, ${\text{S}}$ etc is $\gamma - {\text{S}}{{\text{O}}_3}$.
Thus, the correct option is (C) $\gamma - {\text{S}}{{\text{O}}_3}$.
Note: Sulphur trioxide $\left( {{\text{S}}{{\text{O}}_3}} \right)$ has many structural changes and thus, it has many different forms. These changes are caused by the traces of water. $\gamma - {\text{S}}{{\text{O}}_3}$ is very pure gaseous sulphur trioxide. It is a trimeric structure i.e. it has three molecules of sulphur trioxide. $\gamma - {\text{S}}{{\text{O}}_3}$ forms a cyclic structure. It is colourless solid in appearance. Sulphur trioxide is highly hygroscopic in nature i.e. it can easily attract water molecules from its surroundings by absorption or adsorption.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




